1 Markets
1.1 Market Scope
1.1.1 Key Questions Answered in the Report
1.2 Research Methodology
1.2.1 Next-Generation IVD Market: Research Methodology
1.2.2 Data Sources
1.2.2.1 Primary Data Sources
1.2.2.2 Secondary Data Sources
1.2.3 Market Estimation Model
1.2.4 Criteria for Company Profiling
2 Market Overview
2.1 Introduction
2.1.1 Next-Generation IVD Market Outlook
2.1.1.1 POC-IVD
2.1.1.2 Diagnostic Expenditures
2.1.1.3 Pricing Patterns of Next-Generation IVD
2.1.2 Market Size and Growth Potential
2.1.2.1 Short-Term Impact (2020-2025)
2.1.2.2 Long-Term Impact (2026-2033)
2.1.3 Product Benchmarking by Next- Generation In-vitro Diagnostics Market, (by Type)
2.2 COVID-19 Impact on the Next-Generation IVD Market
2.2.1 Impact on Operations
2.2.2 COVID-19 Impact: Current Scenario of the Market
2.2.3 Pre-COVID Assessment
2.2.4 Post-COVID-19 Market Assessment
3 Industry Analysis
3.1 Legal Requirements
3.1.1 Legal Requirements and Framework in Europe
4 Market Dynamics
4.1 Overview
4.2 Impact Analysis
4.3 Market Drivers
4.3.1 Technological Advancements in Next-Generation In-Vitro Diagnostics Transforming Healthcare System
4.3.2 Rising Demand of POC Testing Boosting Next-Generation IVD Market
4.3.3 Growing Incidence of Chronic and Acute Diseases Demanding for Early Treatment
4.3.4 Boost in the Next-Generation IVD Market during COVID-19 Pandemic
4.4 Market Challenges
4.4.1 Regulatory Hurdles Related to Next-Generation IVD Technologies
4.4.2 Poor Reimbursement Scenario
4.5 Market Opportunities
4.5.1 Rising Number of Next-Generation IVD Companies Involved in Business Expansion
4.5.2 Inclination of Emerging Companies toward Next-Generation IVD
5 Next-Generation IVD Market, by Region, $Million, 2022-2033
5.1 Overview
5.2 Europe
5.2.1 Market Dynamics
5.2.2 Market Size and Forecast
5.2.2.1 Europe Next-Generation IVD Market (by End User)
5.2.2.2 Europe Next-Generation IVD Market (by Type)
5.2.2.2.1 Europe Next-Generation Core Laboratory IVD Market, (by Type)
5.2.2.3 Europe Next-Generation IVD Market (by Country)
5.2.2.3.1 Germany
5.2.2.3.1.1 Market Size and Forecast
5.2.2.3.1.1.1 Germany Next-Generation IVD Market (by End User)
5.2.2.3.1.1.2 Germany Next-Generation IVD Market (by Type)
5.2.2.3.2 France
5.2.2.3.2.1 Market Size and Forecast
5.2.2.3.2.1.1 France Next-Generation IVD Market (by End User)
5.2.2.3.2.1.2 France Next-Generation IVD Market (by Type)
5.2.2.3.3 U.K.
5.2.2.3.3.1 Market Size and Forecast
5.2.2.3.3.1.1 U.K. Next-Generation IVD Market (by End User)
5.2.2.3.3.1.2 U.K. Next-Generation IVD Market (by Type)
5.2.2.3.4 Italy
5.2.2.3.4.1 Market Size and Forecast
5.2.2.3.4.1.1 Italy Next-Generation IVD Market (by End User)
5.2.2.3.4.1.2 Italy Next-Generation IVD Market (by Type)
5.2.2.3.5 Spain
5.2.2.3.5.1 Market Size and Forecast
5.2.2.3.5.1.1 Spain Next-Generation IVD Market (by End User)
5.2.2.3.5.1.2 Spain Next-Generation IVD Market (by Type)
5.2.2.3.6 Rest-of-Europe
5.2.2.3.6.1 Market Size and Forecast
5.2.2.3.6.1.1 Rest-of-Europe Next-Generation IVD Market (by End User)
5.2.2.3.6.1.2 Rest-of-Europe Next-Generation IVD Market (by Type)
6 Company Profiles
6.1 Overview
6.2 bioMérieux SA (BioFire Diagnostics)
6.2.1 Company Overview
6.2.2 Role of bioMérieux SA (BioFire Diagnostics) in the Next-Generation IVD Market
6.2.3 Financials
6.2.4 Recent Developments
6.2.4.1 Corporate Strategies
6.2.4.2 Business Strategies
6.2.5 Analyst Perspective
6.3 F. Hoffmann-La Roche Ltd. (Roche Molecular Systems, Inc.)
6.3.1 Company Overview
6.3.2 Role of F. Hoffmann-La Roche Ltd. in the Next-Generation IVD Market
6.3.3 Financials
6.3.4 Recent Developments
6.3.4.1 Business Strategies
6.3.5 Analyst Perspective
6.4 QIAGEN N.V.
6.4.1 Company Overview
6.4.2 Role of QIAGEN N.V. in the Next-Generation IVD Market
6.4.3 Financials
6.4.4 Recent Developments
6.4.4.1 Corporate Strategies
6.4.4.2 Business Strategies
6.4.5 Analyst Perspective
6.5 Siemens Healthineers AG
6.5.1 Company Overview
6.5.2 Role of Siemens Healthineers AG in the Next-Generation IVD Market
6.5.3 Financials
6.5.4 Recent Developments
6.5.4.1 Corporate Strategies
6.5.4.2 Business Strategies
6.5.5 Analyst Perspective
List of Figures
Figure 1: Facts about Europe Next-Generation IVD Market
Figure 2: Equipment’s used for Next-Generation In-Vitro Diagnostics
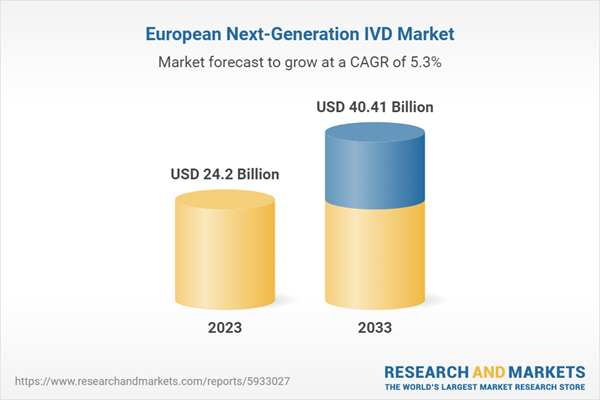
Figure 3: Europe Next-Generation IVD Market, $Billion, 2022 and 2033
Figure 4: Trend Analysis of Next-Generation In-vitro Diagnostics Ecosystem
Figure 5: Common Chronic Conditions for Population Above 65 years
Figure 6: Key Challenges in the Europe Next-Generation IVD Market based on Primary Respondents
Figure 7: New In-Vitro Diagnostics Regulations (IVDR) and Medical Device Regulation (MDR)
Figure 8: Major Highlights Witnessed in the Next-Generation IVD Market During COVID-19 Impact
Figure 9: Role of POC Diagnostics in Next-Generation IVD Market During Pandemic
Figure 10: Leading and Emerging Companies in the Next-Generation IVD Ecosystem
Figure 11: Leading Companies in the Next-Generation IVD Ecosystem and Their Market Share (%)
Figure 12: Key Developments Undertaken by Companies in the Next-Generation IVD Market, July 2019-June 2023
Figure 13: Product Benchmarking of Next-Generation In-Vitro Diagnostics Ecosystem (by Test)
Figure 14: Europe Next-Generation IVD Market (by Type), Share (%), 2022 and 2033
Figure 15: Europe Next-Generation IVD Market (by End User), Share (%), 2022 and 2033
Figure 16: Europe Next-Generation IVD Market, Market Snapshot
Figure 17: Next-Generation IVD Market: Research Methodology
Figure 18: Primary Research Methodology
Figure 19: Bottom-Up Approach (Segment-Wise Analysis)
Figure 20: Top-Down Approach (Segment-Wise Analysis)
Figure 21: Europe Next-Generation IVD Market, $Billion, 2022-2033
Figure 22: Product Benchmarking of Next-Generation In-vitro Diagnostics Market (by Type)
Figure 23: In-Vitro Diagnostic Trends during the COVID-19 Pandemic
Figure 24: Timeline of IVDR 2017/746
Figure 25: Next-Generation IVD Market Dynamics
Figure 26: Factors Contributing to Growing Demand of POC Testing
Figure 27: Current Trend in In-Vitro Diagnostics: From Centralized Laboratory to Point-of-Care Testing
Figure 28: Annual Mortality by Chronic Illness in 1990, 2002, and 2020
Figure 29: Trend in COVID-19 Diagnostic Test Development and the Used Technology
Figure 30: Top Challenges in the IVDR Compliance
Figure 31: Key Area of Focus by Next-Generation IVD Companies
Figure 32: Next-Generation IVD Market (by Region), Share (%), 2022
Figure 33: Next-Generation IVD Market (by Region), $Billion, 2022-2033
Figure 34: Europe Next-Generation IVD Market, $Billion, 2022-2033
Figure 35: Europe Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 36: Europe Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 37: Europe Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 38: Europe Next-Generation IVD Market (by Country), $Billion, 2022-2033
Figure 39: Germany Next-Generation IVD Market, $Billion, 2022-2033
Figure 40: Germany Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 41: Germany Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 42: Germany Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 43: France Next-Generation IVD Market, $Billion, 2022-2033
Figure 44: France Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 45: France Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 46: France Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 47: U.K. Next-Generation IVD Market, $Billion, 2022-2033
Figure 48: U.K. Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 49: U.K. Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 50: U.K. Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 51: Italy Next-Generation IVD Market, $Billion, 2022-2033
Figure 52: Italy Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 53: Italy Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 54: Italy Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 55: Spain Next-Generation IVD Market, $Billion, 2022-2033
Figure 56: Spain Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 57: Spain Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 58: Spain Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 59: Rest-of-Europe Next-Generation IVD Market, $Billion, 2022-2033
Figure 60: Rest-of-Europe Next-Generation IVD Market (by End User), $Billion, 2022-2033
Figure 61: Rest-of-Europe Next-Generation IVD Market (by Type), $Billion, 2022-2033
Figure 62: Rest-of-Europe Next-Generation Core Laboratory IVD Market (by Type), $Billion, 2022-2033
Figure 63: Total Number of Companies Profiled
Figure 64: bioMérieux SA (BioFire Diagnostics): Product Portfolio
Figure 65: bioMérieux SA (BioFire Diagnostics): Overall Financials, $Million, 2020-2022
Figure 66: bioMérieux SA (BioFire Diagnostics): Revenue (by Segment), $Million, 2020-2022
Figure 67: bioMérieux SA (BioFire Diagnostics): Revenue (by Region), $Million, 2020-2022
Figure 68: bioMérieux SA (BioFire Diagnostics): R&D Expenditure, $Million, 2020-2022
Figure 69: F. Hoffmann-La Roche Ltd.: Product Portfolio
Figure 70: F. Hoffmann-La Roche Ltd.: Overall Financials, $Million, 2020-2022
Figure 71: F. Hoffmann-La Roche Ltd.: Revenue (by Segment), $Million, 2020-2022
Figure 72: F. Hoffmann-La Roche Ltd.: Revenue (by Region), $Million, 2020-2022
Figure 73: F. Hoffmann-La Roche Ltd.: R&D Expenditure, $Million, 2020-2022
Figure 74: QIAGEN N.V.: Product Portfolio
Figure 75: QIAGEN N.V.: Overall Financials, $Million, 2020-2022
Figure 76: QIAGEN N.V.: Revenue (by Segment), $Million, 2020-2022
Figure 77: QIAGEN N.V.: Revenue (by Region), $Million, 2020-2022
Figure 78: QIAGEN N.V.: R&D Expenditure, $Million, 2020-2022
Figure 79: Siemens Healthineers AG: Product Portfolio
Figure 80: Siemens Healthineers AG: Overall Financials, $Million, 2020-2022
Figure 81: Siemens Healthineers AG: Revenue (by Segment), $Million, 2020-2022
Figure 82: Siemens Healthineers AG: Revenue (by Region), $Million, 2020-2022
Figure 83: Siemens Healthineers AG: R&D Expenditure, $Million, 2020-2022
List of Tables
Table 1: Impact Analysis of Market Drivers, and Restraints on the Europe Next-Generation IVD Market
Table 2: Impact of COVID-19 on Different Countries
Table 3: Likert Scale
Table 4: Impact Analysis of Market Drivers
Table 5: Impact Analysis of Market Restraints
Table 6: Impact Analysis of Market Opportunities
Table 7: Technology Offered by Next-Generation IVD Ecosystem
Table 8: Examples of Expansion Strategy by Next-Generation IVD Market Players
Table 9: Europe: Market Dynamics