Free Webex Call
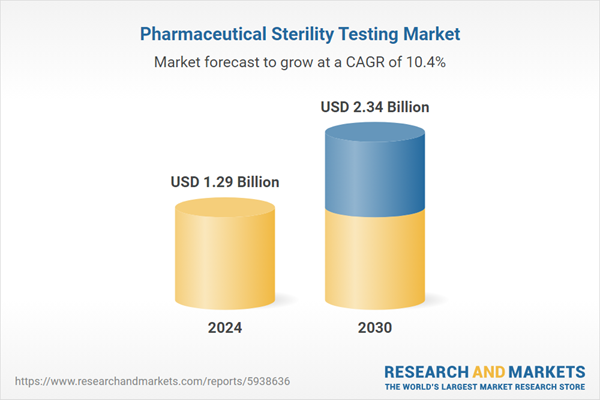
Global Pharmaceutical Sterility Testing Market was valued at USD 1.29 billion in 2024 and is expected to reach USD 2.34 billion by 2030 with a CAGR of 10.43% during the forecast period. Pharmaceutical sterility testing is a critical quality control process used in the pharmaceutical industry to ensure that a pharmaceutical product is free from viable microorganisms, including bacteria, fungi, yeast, and other potentially harmful contaminants. The primary objective of sterility testing is to verify that a pharmaceutical product is sterile and safe for patient use. Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Biotechnology Advancements
Biotechnology has introduced molecular biology methods, such as Polymerase Chain Reaction (PCR) and real-time PCR, for the detection of specific microbial DNA or RNA in samples. These techniques allow for rapid and highly specific identification of microorganisms, improving the speed and accuracy of sterility testing. Next-Generation Sequencing (NGS) technology has revolutionized microbial identification and genotypic characterization. It enables comprehensive microbial profiling and can detect a wide range of microorganisms in a single test. NGS can be applied to environmental monitoring and identifying contaminants in pharmaceutical products. Microarrays allow for the simultaneous detection of multiple microorganisms in a single test.They are especially valuable for environmental monitoring and identifying potential sources of contamination in pharmaceutical manufacturing facilities. Flow cytometry, coupled with fluorescent staining techniques, can rapidly assess microbial viability and cell count. This technology is useful for both in-process monitoring and the final product sterility testing. Biotechnology has enhanced traditional culture methods. Automated microbial detection systems, using technologies like bioluminescence or impedance, speed up the detection process and provide a real-time assessment of microbial growth. Biotechnology has enabled high-throughput sterility testing systems, which can process many samples simultaneously. This is particularly valuable for pharmaceutical manufacturers dealing with a high volume of products.
Microfluidic devices allow for miniaturized and automated sterility testing systems. They enable precise handling of small sample volumes and can reduce the time required for testing. Advances in bioinformatics and data analysis tools help in interpreting the vast amount of data generated by modern sterility testing methods. These tools facilitate microbial identification and genotypic characterization. Biotechnology has facilitated the development of portable and rapid sterility testing devices, which can be used in field applications and remote locations, enhancing access to sterility testing in resource-limited settings.
Biotechnology has enabled the development of single-use sterility testing systems, reducing the risk of cross-contamination, and improving the efficiency of testing in pharmaceutical manufacturing. Biotechnology advancements have also contributed to the quality control and validation of sterility testing processes. This includes the development of reference materials, standards, and proficiency testing programs to ensure the accuracy and reliability of testing. Biotechnology-driven automation, including robotics, has enhanced the sterility testing process, reducing the potential for human error, and improving overall efficiency. This factor will help in the development of the Global Pharmaceutical Sterility Testing Market.
Key Market Challenges
Complex and Biologic Drug Products
Complex and biologic drug products, such as monoclonal antibodies and gene therapies, can be sensitive to traditional sterilization methods like heat or radiation. Sterility testing methods must be adapted to ensure that the product's efficacy is not compromised during the testing process. Cell-based therapies, such as stem cell treatments, are considered biologic drug products. Sterility testing for these therapies must address the presence of live cells, making traditional methods less suitable. Specialized techniques are required to detect potential contamination without harming the therapeutic cells. Some complex drug products have intricate formulations, often involving liposomes, nanoparticles, or emulsions.These complex formulations can interfere with traditional sterility testing methods, requiring the development of novel techniques to ensure accurate results. Biologic drug products, especially those derived from cell cultures, are at risk of viral contamination. Sterility testing for these products includes specific tests to detect the presence of viral contaminants. Some biologic drugs are administered in extremely low dosages. The sensitivity and accuracy of sterility testing methods become critical to detect any potential contamination in such small quantities.
Key Market Trends
Advancements in Aseptic Processing
Pharmaceutical manufacturers are increasingly adopting advanced barrier systems, including isolators and Restricted Access Barrier Systems (RABS). These systems create physical barriers that prevent external contaminants from entering the manufacturing process. These technologies improve sterility assurance during drug product filling and packaging. Single-use systems, such as disposable bags, tubing, and connectors, are being integrated into aseptic processing. They reduce the risk of cross-contamination and simplify the cleaning and sterilization processes, thereby enhancing sterility control. Automation and robotics play a vital role in aseptic processing by minimizing human intervention and reducing the potential for contamination.Automated filling, capping, and inspection systems enhance product sterility and consistency. Innovations in fill-finish technologies, such as pre-sterilized containers, nested syringes, and ready-to-use vials, are simplifying the aseptic filling process. These advancements reduce the handling of components and minimize the risk of contamination. Advanced microbial detection methods, including rapid microbial methods (RMM), are being integrated into aseptic processing to allow for real-time monitoring of microbial contamination. This enables proactive responses and corrective actions.
Key Market Players
- Pacific BioLabs Inc.
- STERIS
- Boston Analytical
- Nelson Laboratories, LLC
- Sartorius AG
- SOLVIAS AG
- Charles River Laboratories
- Thermo Fisher Scientific, Inc.
- Rapid Micro Biosystems, Inc.
- Almac Group
Report Scope:
In this report, the Global Pharmaceutical Sterility Testing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Pharmaceutical Sterility Testing Market, By Type:
- In-house
- Outsourcing
Pharmaceutical Sterility Testing Market, By Product Type:
- Kits and Reagents
- Instruments
- Service
Pharmaceutical Sterility Testing Market, By Test Type:
- Sterility Testing
- Membrane filtration
- Direct inoculation
- Bioburden Testing
- Bacterial Endotoxin Testing
Pharmaceutical Sterility Testing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Pharmaceutical Sterility Testing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
1. Product Overview
2. Research Methodology
3. Executive Summary
5. Global Pharmaceutical Sterility Testing Market Outlook
6. Asia Pacific Pharmaceutical Sterility Testing Market Outlook
7. Europe Pharmaceutical Sterility Testing Market Outlook
8. North America Pharmaceutical Sterility Testing Market Outlook
9. South America Pharmaceutical Sterility Testing Market Outlook
10. Middle East and Africa Pharmaceutical Sterility Testing Market Outlook
11. Market Dynamics
12. Market Trends & Developments
14. Porter’s Five Forces Analysis
16. Competitive Landscape
Companies Mentioned
- Pacific BioLabs Inc.
- STERIS
- Boston Analytical
- Nelson Laboratories, LLC
- Sartorius AG
- SOLVIAS AG
- Charles River Laboratories
- Thermo Fisher Scientific, Inc.
- Rapid Micro Biosystems, Inc.
- Almac Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.29 Billion |
| Forecasted Market Value ( USD | $ 2.34 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |