Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
It involves the use of automated systems and instruments for tasks such as cell culture, fermentation, purification, and fill-finish operations. Process automation ensures the precise control of critical parameters (e.g., temperature, pH, and agitation) to maintain product quality and consistency. Robotic systems are employed for a wide range of tasks, from drug discovery and sample handling to filling and packaging in manufacturing. Robots can be programmed to handle samples, reagents, and labware, performing tasks with precision and repeatability.
Key Market Drivers
Technological Advancements
Robotics and automation have become increasingly sophisticated, with the integration of advanced robotics in laboratories and production facilities. These robots can perform a wide range of tasks, from liquid handling and sample preparation to cell culture maintenance. Automation systems have improved the efficiency of High-Throughput Screening (HTS) in drug discovery. Automated liquid handling and data analysis tools allow for the rapid screening of thousands of compounds for potential drug candidates. Lab-on-a-Chip (LOC) Technology integrates various laboratory functions onto a single chip, reducing the need for manual interventions.This technology is particularly useful for sample preparation and analysis. Advancements in software and data analytics enable real-time monitoring and analysis of experimental data. This facilitates quicker decision-making in drug development and manufacturing processes. Artificial Intelligence (AI) and Machine Learning (ML) are being used for data analysis, predictive modeling, and optimization of bioprocessing. They can help identify potential drug candidates and optimize manufacturing processes.
Key Market Challenges
Cost of Implementation
The purchase of automation equipment, software, and related technologies requires a substantial capital investment. This includes the costs of acquiring hardware, robotics, sensors, and other automation components. Tailoring automation systems to the specific needs of biopharmaceutical processes often involves customization. Custom solutions are typically more expensive than off-the-shelf products. Integrating automation systems with existing processes and infrastructure can be complex and costly. Compatibility and interoperability with legacy systems may require additional investments.Ensuring that automation systems meet regulatory standards and industry-specific validation requirements adds to the cost. Extensive testing, documentation, and validation processes are necessary to ensure compliance. Employees must be trained to operate and maintain automation systems effectively. Training programs come with associated costs, including staff time and external training resources. Automation systems require ongoing maintenance and technical support. This includes software updates, equipment maintenance, and troubleshooting, all of which have associated costs.
Key Market Trends
Lab Automation
Lab automation systems enable high-throughput screening of compounds, allowing researchers to quickly test many potential drug candidates. This is essential in the early stages of drug discovery. Automation systems in the lab provide precise and accurate handling of samples, reducing the risk of human error and ensuring consistent and reliable results. Automation simplifies data collection, storage, and analysis. It enables real-time monitoring and data processing, facilitating faster decision-making in research and development.Automated processes are more efficient and can run continuously, reducing the time and effort required for experiments and assays. This accelerates research and development timelines. Automation reduces the need for manual labor in repetitive and time-consuming tasks, resulting in cost savings and the redeployment of human resources to more strategic activities. Automation optimizes the use of resources, such as reagents, consumables, and equipment, making laboratory operations more cost-effective.
ABB Robotics will return for a second year to the Society for Laboratory Automation and Screening (SLAS) 2025 International Conference & Exhibition, January 25-29 at the San Diego Convention Center (Booth #2541). Highlights of ABB’s participation include two live robotic automation demos and a roundtable discussion with industry leaders, including METTLER TOLEDO and Agilent Technologies, to explore the challenges laboratories face and how robotics can help, on January 28.
Key Market Players
- PerkinElmer, Inc.
- AMETEK, Inc.
- Autodesk, Inc.
- Baumueller-Nuermont Corp
- Emerson Electric Co.
- Kawasaki Robotics
- RheoSense Inc.
- Rockwell Automation, Inc.
- Sartorius Stedim Biotech SA
- Siemens Healthineers
Report Scope:
In this report, the Global Automation in Biopharma Industry Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Automation in Biopharma Industry Market, By Technology:
- Automation Technology
- Digitization Technology
Automation in Biopharma Industry Market, By Application:
- Clinical Phase
- Drug Discovery Phase
- Production Phase
Automation in Biopharma Industry Market, By Component:
- Automation Hardware
- Automation Software
- Services Project Phase
- Services Operation Phase
Automation in Biopharma Industry Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Automation in Biopharma Industry Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- PerkinElmer, Inc.
- AMETEK, Inc.
- Autodesk, Inc.
- Baumueller-Nuermont Corp
- Emerson Electric Co.
- Kawasaki Robotics
- RheoSense Inc.
- Rockwell Automation, Inc.
- Sartorius Stedim Biotech SA
- Siemens Healthineers
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
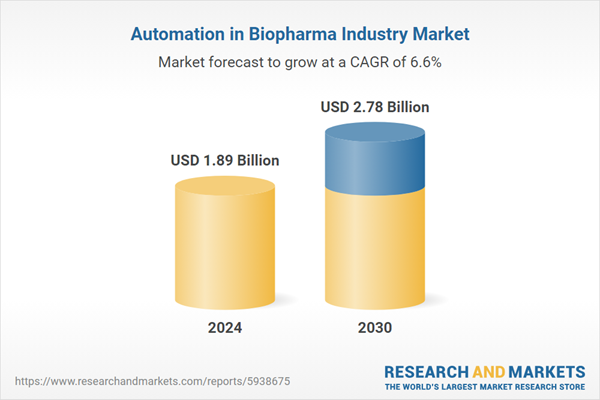
| Estimated Market Value ( USD | $ 1.89 Billion |
| Forecasted Market Value ( USD | $ 2.78 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |