Sandhoff Disease: Introduction
Sandhoff disease, a rare inherited disorder, gradually devastates nerve cells within the central nervous system, encompassing the brain and spinal cord. It is categorized into three primary types, distinguished by the onset of signs and symptoms: infantile, juvenile, and adult variants. Among these, the infantile manifestation stands as the most prevalent and severe, manifesting during infancy itself. Typically, infants affected by this form of the disease seem healthy until around 3 to 6 months of age, after which a noticeable decline in their developmental progress occurs, accompanied by weakening of movement-related muscles.Sandhoff Disease Market Analysis
The market growth is driven by ongoing research and development activities that are focused on discovering the best treatment approach for this life-threatening rare genetic disease. The increasing awareness about Sandhoff disease is a major factor that individuals are now asking for effective treatment, propelling the market demand. Since the market does not have a full-proof effective treatment available, the market growth may be restrained in the forecast period.Furthermore, the dedicated actions by the doctors and researchers are exhibiting a rigid determination toward finding a cure for this condition. Sandhoff disease is a rare disorder that slowly destroys the nerve cells in the nervous system resulting in patient losing their abilities to sit, see, and sense collectively. Children suffering from this disease often die at an age not more than three years.
Such crucial yet heartbreaking facts are prominent to consider for market players and doctors to find a solution for this rare disorder which will result in increased research and development, activities, FDA approvals, and clinical trials, expected to bolster the market growth and relieve the patients suffering from the medical condition.
Sandhoff Disease Market Segmentations
Sandhoff Disease Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Infantile
- Juvenile
- Late Onset
Market Breakup by Therapy
- Gene Therapy
- Enzyme Replacement Therapy
- Stem Cell Therapy
- Others
Market Breakup by Treatment
- Medication
- Surgery
Market Breakup by Drugs
- Anticonvulsants
- Miglustat
- Others
Market Breakup by Route of Administration
- Oral
- Inhalation
- Parenteral
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
Sandhoff Disease Market Overview
The market is expected to experience significant growth driven by factors such as increasing awareness among individuals regarding genetic disorders and the increasing allocation of special drug designations by regulatory authorities is anticipated to drive market growth. Moreover, intensifying competition among pharmaceutical companies is acting as a catalyst in boosting market growth. Nonetheless, obstacles such as limited accessibility to essential services in remote regions and insufficient training and knowledge among healthcare professionals are potentially expected to restrain growth. The increasing number of investments from the biotechnology and pharmaceutical industries into research and development initiatives are expected to bolster the Sandhoff disease market growth in the forecast period.The United States is currently leading the market and is expected to continue dominating the market in the forecast period. The regional market growth can be attributed to advanced treatment availability and the presence of a robustly developed healthcare infrastructure. Other regions are also likely to witness significant growth in the coming years owing to its steady advancement in healthcare infrastructure and escalating governmental investments in research and development.
Sandhoff Disease Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- NeoImmuneTech
- Neurimmune
- GlaxoSmithKline Plc
- Sanofi SA
- Gilead Sciences
- Allergen Plc
- Novatris AG
- Abbvie Inc.
- Bristol-Myers Squibb Company
- Akero Therapeutics, Inc.
- Bristol-Myers Squibb Company
- Alexion Pharmaceuticals, Inc
- Grifols
- Intellia Therapeutics
- Ionis Pharmaceuticals, Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- NeoImmuneTech
- Neurimmune
- GlaxoSmithKline Plc
- Sanofi SA
- Gilead Sciences
- Allergen Plc
- Novatris AG
- Abbvie Inc.
- Bristol-Myers Squibb Company
- Akero Therapeutics, Inc.
- Alexion Pharmaceuticals, Inc
- Grifols
- Intellia Therapeutics
- Ionis Pharmaceuticals, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
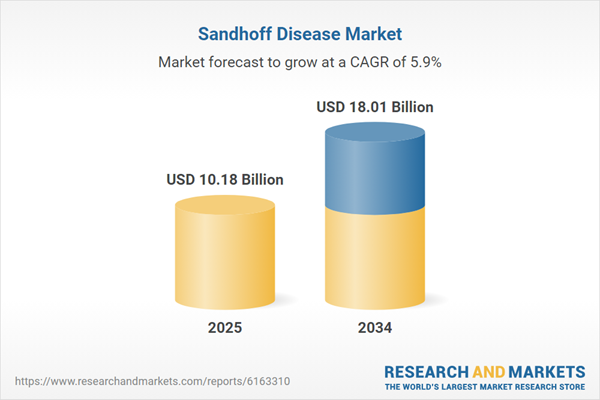
| Estimated Market Value ( USD | $ 10.18 Billion |
| Forecasted Market Value ( USD | $ 18.01 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |