Vietnam Interventional Cardiology Market Overview
The market is undergoing dynamic growth due to various factors contributing to the evolution of cardiovascular care within the country. With a surge in the prevalence of cardiovascular diseases and an aging population, there is a heightened demand for sophisticated interventional cardiology devices. These include catheters, stents, and guidewires, among others. As Vietnam's healthcare infrastructure continues to improve, there is a notable increase in awareness regarding cardiac health, leading to a growing adoption of interventional procedures. Government initiatives to enhance overall healthcare services are playing a pivotal role in shaping the landscape of interventional cardiology in Vietnam. The ongoing efforts to modernize medical facilities and the promotion of preventive healthcare measures are also likely to contribute significantly to the Vietnam interventional cardiology market share.Moreover, the rising trend of minimally invasive procedures is gaining momentum, driven by factors such as reduced recovery times and lower associated risks. Major industry players are actively contributing to market growth by introducing innovative technologies tailored to the specific needs of the Vietnamese population. The collaborative efforts between healthcare providers and industry stakeholders further enhance accessibility to state-of-the-art interventional cardiology devices, fostering a conducive environment for advancements in cardiac care.
Rising Prevalence of Cardiovascular Diseases
In Vietnam, the rising prevalence of cardiovascular diseases has spurred a significant demand for advanced medical interventions, particularly in the field of interventional cardiology devices. The increase in lifestyle-related risk factors, such as sedentary habits and dietary changes, has contributed to a rising incidence of cardiac ailments. To address this growing health concern, the Vietnamese healthcare sector is increasingly adopting interventional cardiology devices. These innovative technologies, including stents, catheters, and angioplasty devices, play a pivotal role in diagnosing and treating cardiovascular conditions.The Vietnam interventional cardiology market growth can be attributed to the proactive approach in the region to combat the escalating burden of heart diseases, emphasizing the importance of timely and effective interventions to enhance patient outcomes and overall cardiovascular health in the region. Based on data presented in the EvoHealth White Paper on ASCVD in Vietnam, the year 2022 witnessed a total of 2.4 million individuals grappling with cardiovascular diseases, and a significant 65% of these cases were attributed to atherosclerosis. Notably, atherosclerosis emerged as the predominant cause of mortality, exhibiting a notably elevated incidence of ischemic heart disease and ischemic stroke.
Adoption of Artificial Intelligence (AI) to Propel the Interventional Cardiology Market Demand in Vietnam
In January 2023, clinical studies revealed that digitally facilitated cardiovascular interventions exhibit the potential to enhance patient outcomes, resulting in a notable 23% decrease in hospital re-admissions. Emerging technologies in the cardiac field encompass artificial intelligence (AI), big data, blockchain, Alexa skills, and chatbots. AI, a groundbreaking technology, enables the processing of extensive data through algorithms, aiding healthcare professionals in optimal decision-making for patient well-being. Modern cardiac ultrasound systems now integrate AI to autonomously identify, segment, label anatomy, determine optimal echo views, and conduct automatic measurements, streamlining the diagnostic process for cardiologists. Such developments are likely to contribute significantly to the Vietnam interventional cardiology market share.
Presently, AI-driven calcium scoring software for cardiac CT scans swiftly generates quantification information and color-codes calcium by vessel segment on dataset slices. AI algorithms are employed to autonomously identify arrhythmias, issuing alerts to patients via wearables or smartphone-based apps that record ECG data. Notably, studies have demonstrated the Kardia, when paired with an Apple Watch, exhibits high sensitivity in detecting atrial fibrillation.
Additionally, research indicates that, when integrated with AI, this technology can non-invasively identify elevated blood potassium levels, potentially indicating diabetes or heart failure. Devices like the Apple Watch and the Kardia Alivecor exemplify such AI applications. Cardiology is experiencing significant strides in AI development, particularly in point-of-care triage through apps and wearable cardiac monitoring technologies. This advancement is poised to enhance the examination process for high-risk patients, facilitating earlier detection of cardiovascular diseases by human cardiology specialists.
Vietnam Interventional Cardiology Market Segmentation
Vietnam Interventional Cardiology Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Catheters
- Angiography Catheters
- Balloon Dilatation Catheters
- Percutaneous Transluminal Coronary Angioplasty Guiding Catheters
- Guide Extension Catheters
- Microcatheters
- Dual Lumen Catheters
- Others
- Guide Wires
- Coronary Guide Wires
- Specialty Guide Wires
- Coronary Stents
- Bare Stents
- Drug-Eluting Stents
- Bio Absorbable Stents
- Angioplasty Balloons
- Old/Normal Balloons
- Drug-Eluting Balloons
- Cutting/Scoring Balloons
- Plague Modification Devices
- Thrombectomy Devices
- Atherectomy
- Hemodynamic Flow Alteration Devices
- Embolic Protection Devices
- Chronic Total Occlusion Devices
- Vascular Closure Devices (VCDS)
- Nitinol Clip-Based (Starclose) Devices
- Suture Based (Perclose)
- Collagen Plug-Based (Angio-Seal)
- Fractional Flow Reserve (FFR)
- Accessories
- Angioplasty Kits
- Balloon Inflation Devices
- Introducer Sheaths
- Hemostasis Heart
- Others
Market Breakup by Type
- Conventional
- Catheters
- Coronary Guide Wires
- Coronary Stents
- Vascular Closure Devices (VCDS)
- Others
- Advanced
- Plague Modification Devices
- Hemodynamic Flow Alteration Devices
- Intravascular Ultrasound (IVUS) Catheters
- Fractional Flow Reserve (FFR)
Market Breakup by Indication
- Cardiac Catheterization
- Percutaneous Coronary Intervention (PCI)
- Coronary Thrombectomy
- Atherectomy
- Balloon Angioplasty
- Stent Implantation
- Hypothermia/Intra-Aortic Balloon Pump
- Patent Foramen Ovale Closure
- Others
Market Breakup by Age
- Adult
- Pediatric
- Geriatric
Market Breakup by Distribution Channel
- Direct Tenders
- Third Party Distributors
Market Breakup by End User
- Hospitals
- Acute Care Hospitals
- Long-Term Care Hospitals
- Cath Laboratories
- Academic and Research Institutes
Market Breakup by Region
- Northern and Central
- Western
- Southern
- Eastern
Vietnam Interventional Cardiology Market: Competitor Landscape
In December 2023, Sweef Capital revealed the second investment from its Southeast Asia Women's Economic Empowerment Fund (SWEEF) in USM Healthcare (USM). USM is the pioneer company in Vietnam dedicated to local production of essential cardiovascular products.The key features of the interventional cardiology market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players.
The competitive landscape of the interventional cardiology market in Vietnam offers comprehensive information about each competitor. This includes company overview, financials, revenue generation, market potential, research and development investments, new market initiatives, production sites, strengths and weaknesses, product launches, product trial pipelines, product approvals, patents, product range, application dominance, and technology life cycle. It is important to note that these data points specifically pertain to the company's involvement in the Vietnam interventional cardiology market.
The major companies in the market are as follows:
- Boston Scientific Corporation
- Terumo Vietnam Medical Equipments Co., Ltd
- Abbott Laboratories
- Medtronic Plc
- Stryker Corporation
- Becton, Dickinson and Company
- Angio Dynamics
- B. Braun Melsungen AG
- Biotronik
- Medinol LTD.
- Philips Electronics N.V.
- Teleflex Incorporated
- USM Healthcare
- Edwards Lifesciences
- Penumbra Inc
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- Terumo Vietnam Medical Equipments Co., Ltd
- Abbott Laboratories
- Medtronic Plc
- Stryker Corporation
- Becton, Dickinson and Company
- Angio Dynamics
- B. Braun Melsungen AG
- Biotronik
- Medinol LTD.
- Philips Electronics N.V.
- Teleflex Incorporated
- USM Healthcare
- Edwards Lifesciences
- Penumbra Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
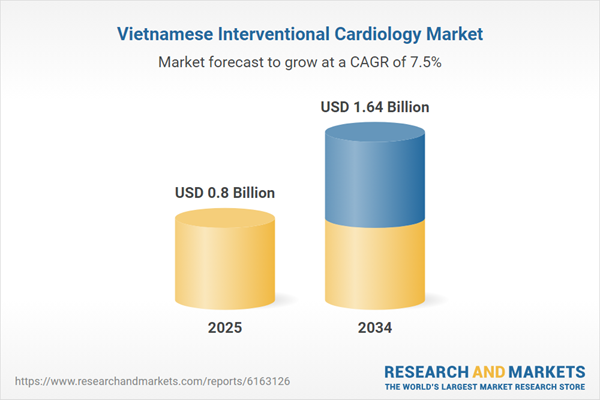
| Estimated Market Value ( USD | $ 0.8 Billion |
| Forecasted Market Value ( USD | $ 1.64 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Vietnam |
| No. of Companies Mentioned | 15 |