Hypodermic Syringes and Needles: Introduction
A hypodermic needle is used to inject substances like medications or fluids into the body or extract fluids out of it. Hypodermic needles are hollow and are mostly used with a syringe. It offers rapid delivery of fluids and is widely used when a substance cannot be ingested orally. It also reduces the risk of contamination by preventing entrapment of airborne pathogens and by preventing the microbes' entry into the substrate.Latin America Hypodermic Syringes and Needles Market Analysis
With the sudden outbreak of COVID-19, the medical ecosystem has transformed on many levels. With vaccination being a necessity, many people have become familiar with and comfortable around needles and syringes. Acknowledging the impact of vaccinations, the Latin America hypodermic syringes and needles market demand has grown, as more people proceed to administer vaccinations for other diseases.Owing to significant emphasis on improving one's physical appearance and aesthetics, Brazil is experiencing a boost in the incidence of cosmetic surgeries like rhinoplasty, body contouring, liposuction, lip augmentation, and Botox. This has contributed to the market growth of needles and syringes in the region. The prevalence of other diseases such as diabetes, cardiovascular issues, autoimmune disorders, and overall surgical interventions has also led to higher application of medical equipment.
The Latin America hypodermic syringes and needles market value is also driven by the increasing advances in medical equipment and technology. Needles are now available in a wide range of gauze sizes, lengths, and bevel designs to address different medical conditions in patients. With a vision to reduce medical waste, they are also accompanied by tip caps.
Latin America Hypodermic Syringes and Needles Market Segmentation
Latin America Hypodermic Syringes and Needles Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Conventional (Bevel, Vented)
- Safety
Market Breakup by Products
- Suture
- Blood Collection
- Insufflation
Market Breakup by Material
- Stainless Steel
- Plastic
Market Breakup by Delivery Mode
- IV
- IM
- Hypodermic
Market Breakup by Region
- Brazil
- Argentina
- Mexico
- Others
Latin America Hypodermic Syringes and Needles Market Overview
In Latin America, Brazil holds the largest hypodermic syringes and needles market share and contributes 9.1% of its GDP to healthcare. With a steadily growing medical device market, the region is experiencing a hike in the number of hospital set-ups. E-commerce is a developing channel for medical consumables like hypodermic syringes and needles, fostering the rising demand amongst locals, especially with a rise in the incidence of surgical interventions in the region.Mexico is a significant player in the market with proactive public healthcare institutions working towards benefiting the population. The region has been experiencing a rise in the local production of medical devices and equipment with the market size attaining USD 30.4 billion in 2023. In addition, the overall healthcare ecosystem has been improving, characterised by the drive to reduce costs and offer affordability for the common people.
Argentina is witnessing significant hypodermic syringes and needles market growth owing to rising foreign investments in the region. With 35% of the population relying on public hospitals, there have been some effective measures to improve the public health system. In April 2023, the Inter-American Development Bank (IDB) granted a USD 200 million loan to boost Argentina's healthcare system. Such investments are expected to bolster the hypodermic needle and syringe market directly.
Latin America Hypodermic Syringes and Needles Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Medtronic PLC
- Becton, Dickinson and Company
- Stryker Corporation
- Ethicon, Inc. (Subsidiary of Johnson and Johnson)
- Boston Scientific Corporation
- Terumo Corporation
- Olympus Corporation
- Merit Medical
- Greiner Holding AG
- Merck KgA
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Medtronic PLC
- Becton, Dickinson and Company
- Stryker Corporation
- Ethicon, Inc. (Subsidiary of Johnson and Johnson)
- Boston Scientific Corporation
- Terumo Corporation
- Olympus Corporation
- Merit Medical
- Greiner Holding AG
- Merck KgA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
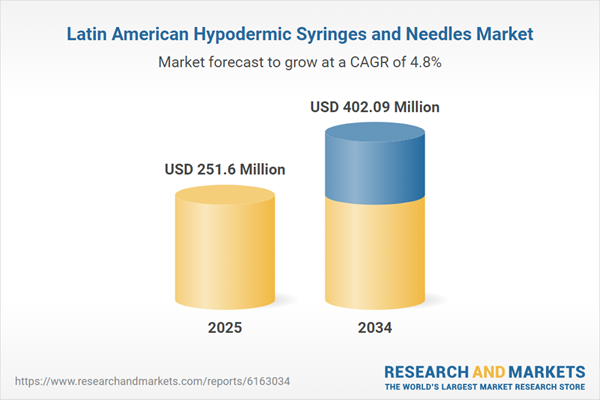
| Estimated Market Value ( USD | $ 251.6 Million |
| Forecasted Market Value ( USD | $ 402.09 Million |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Latin America |
| No. of Companies Mentioned | 10 |