Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Technological advancements in surgical techniques, including minimally invasive procedures, have expanded the indications for sternal closure systems. Minimally invasive approaches require specialized closure systems designed to accommodate smaller incisions and reduced tissue trauma, driving innovation and adoption in the market. The aging population worldwide contributes to the increasing demand for cardiac surgeries and sternal closure procedures. As individuals age, they are more likely to develop cardiovascular diseases and require surgical interventions, driving the growth of the sternal closure systems market. Healthcare providers are increasingly focused on improving patient safety, surgical outcomes, and quality of care. Secure sternal closure is essential for minimizing the risk of post-operative complications such as sternal dehiscence and mediastinitis, leading to the widespread adoption of sternal closure systems.
Key Market Drivers
Advancements in Surgical Techniques
Advancements in surgical techniques have led to the widespread adoption of minimally invasive procedures for various cardiac and thoracic surgeries. Minimally invasive approaches typically involve smaller incisions and reduced tissue trauma compared to traditional open surgeries. As a result, there is a growing demand for sternal closure systems that are specifically designed to accommodate these minimally invasive techniques. Minimally invasive surgical techniques require specialized sternal closure systems that are flexible, easy to use, and capable of providing secure closure while minimizing tissue trauma. These closure systems may include innovative designs, such as sutures, clips, or sternal plates, that offer enhanced stability and support during the healing process. Minimally invasive procedures are associated with a lower risk of post-operative complications, such as sternal dehiscence and mediastinitis, compared to traditional open surgeries. Secure sternal closure is essential for preventing these complications and promoting optimal healing following cardiac and thoracic surgeries. Advancements in surgical techniques, coupled with the use of specialized sternal closure systems, contribute to improved patient outcomes. Minimally invasive approaches are associated with shorter hospital stays, faster recovery times, reduced post-operative pain, and improved cosmetic results compared to traditional open surgeries. These benefits enhance patient satisfaction and quality of life.Surgeons prefer to use sternal closure systems that are compatible with minimally invasive techniques and offer reliable performance. Advanced closure systems that facilitate secure sternal closure and minimize the risk of complications provide surgeons with greater confidence during surgical procedures. Advancements in technology drive innovation in sternal closure systems, leading to the development of new materials, designs, and techniques. Manufacturers continually invest in research and development to improve the effectiveness, safety, and usability of sternal closure systems, aligning with the evolving needs of surgeons and patients. As patients and healthcare providers increasingly recognize the benefits of minimally invasive procedures, there is a growing demand for sternal closure systems that support these techniques. Manufacturers respond to market demand by developing specialized closure systems that address the unique requirements of minimally invasive surgeries. This factor will help in the development of the Global Sternal Closure Systems Market.
Growing Aging Population
As individuals age, they are more susceptible to cardiovascular diseases such as coronary artery disease, valvular heart disease, and congestive heart failure. These conditions often require surgical interventions, including cardiac procedures that involve sternotomy and sternal closure. The increasing prevalence of cardiovascular diseases among the aging population drives the demand for sternal closure systems. The aging population experiences a higher incidence of cardiac conditions that necessitate surgical treatment. As a result, there is a greater need for cardiac surgeries such as coronary artery bypass grafting (CABG), valve repair or replacement, and aortic surgeries, all of which require sternal closure. The growing aging population contributes to the rising demand for sternal closure systems to support these surgical procedures. Older patients often present with multiple comorbidities and complex medical histories, which may increase the complexity of surgical cases. Surgeons may encounter challenges related to sternal closure in older patients, including compromised sternal integrity, reduced bone quality, and increased risk of post-operative complications. The demand for sternal closure systems capable of addressing these challenges is driven by the aging population's need for cardiac surgeries.Advances in healthcare, medical technology, and disease management have led to prolonged life expectancy globally. As individuals live longer, they are more likely to develop age-related cardiovascular conditions that require surgical treatment. The increasing life expectancy of the aging population contributes to the sustained demand for sternal closure systems over time. Older adults are increasingly focused on maintaining their quality of life and participating in activities that enhance their well-being. Cardiac surgeries, including those requiring sternal closure, can improve quality of life by alleviating symptoms, restoring cardiac function, and prolonging life expectancy. The demand for sternal closure systems reflects the aging population's desire to preserve and improve their quality of life through surgical interventions. Healthcare systems worldwide must adapt to the needs of an aging population by investing in infrastructure, resources, and specialized care services. This includes the availability of cardiac surgical facilities, trained healthcare professionals, and access to sternal closure systems that meet the unique needs of older patients undergoing cardiac surgeries. This factor will pace up the demand of the Global Sternal Closure Systems Market.
Emerging Markets and Economic Development
Economic development in emerging markets often leads to higher healthcare expenditure as governments and individuals allocate more resources to healthcare infrastructure, services, and technologies. Increased healthcare spending supports the expansion of cardiac care facilities, the adoption of advanced medical technologies, and the demand for sternal closure systems to support cardiac surgeries. Economic development facilitates the development and improvement of healthcare infrastructure in emerging markets. This includes the establishment of specialized cardiac care centers, hospitals, and surgical facilities equipped with the latest medical equipment and technologies required for cardiac procedures, including sternal closure systems. Emerging markets are experiencing a growing burden of cardiovascular diseases due to factors such as urbanization, changing lifestyles, and aging populations. The increasing prevalence of cardiovascular diseases drives the demand for cardiac surgeries, including those requiring sternal closure, in emerging markets. Economic development enables emerging markets to gain access to advanced medical technologies and innovations, including sternal closure systems. As healthcare infrastructure improves and healthcare providers receive training on the latest surgical techniques and technologies, the adoption of sternal closure systems increases to enhance patient outcomes and safety.Economic development is often accompanied by an increased demand for quality healthcare services among the population in emerging markets. Patients seek access to advanced medical treatments, including cardiac surgeries, to address their healthcare needs and improve their quality of life. The demand for sternal closure systems is driven by the growing expectations for safe, effective, and reliable cardiac procedures. Governments in emerging markets may implement healthcare initiatives and policies aimed at improving access to healthcare services, reducing the burden of disease, and enhancing healthcare outcomes. These initiatives may include subsidies, incentives, and regulations that promote the adoption of advanced medical technologies, including sternal closure systems, in cardiac care. Recognizing the growth potential in emerging markets, manufacturers of sternal closure systems are expanding their presence and distribution networks in these regions. They may tailor their products and pricing strategies to meet the needs and preferences of healthcare providers and patients in emerging markets, driving market growth and penetration. This factor will accelerate the demand of the Global Sternal Closure Systems Market.
Key Market Challenges
Market Saturation and Competition
The market for sternal closure systems is highly competitive, with numerous manufacturers vying for market share. Intense competition leads to price pressure and the need for differentiation through product innovation, quality improvement, and service offerings. In some segments of the market, sternal closure systems may become commoditized, with little differentiation among products in terms of features and functionality. This can lead to price-based competition, eroding profit margins for manufacturers and distributors. Larger companies may engage in consolidation strategies, such as mergers and acquisitions, to gain market dominance and competitive advantage. This consolidation can create barriers to entry for smaller players and limit choice for consumers. Accessing international markets requires navigating complex regulatory frameworks, cultural differences, and varying reimbursement systems. Manufacturers must adapt their strategies to meet the unique requirements of each market while remaining competitive on a global scale. Building and maintaining strong customer relationships is critical in a competitive market environment. Manufacturers must focus on delivering value-added services, fostering loyalty, and differentiating themselves based on customer satisfaction and support.Risk of Adverse Events
Adverse events related to sternal closure systems can compromise patient safety and result in serious complications such as sternal dehiscence, mediastinitis, and wound infections. Ensuring the safety and well-being of patients undergoing cardiac and thoracic surgeries is a primary concern for healthcare providers and manufacturers alike. Poor sternal closure techniques or inadequate sternal fixation can lead to post-operative complications, prolong hospital stays, and increase healthcare costs. Adverse events may necessitate additional medical interventions, including surgical revisions, antibiotic therapy, and wound care management, further burdening patients and healthcare systems. Maintaining quality control and assurance throughout the manufacturing process is essential for minimizing the risk of adverse events associated with sternal closure systems. Manufacturers must adhere to strict quality standards, conduct comprehensive testing, and implement robust quality management systems to ensure product safety and efficacy. Regulatory authorities impose stringent requirements and standards to ensure the safety, effectiveness, and quality of medical devices, including sternal closure systems. Compliance with regulatory guidelines is necessary to obtain market approval and maintain patient trust in the safety and reliability of sternal closure products. Proper training and expertise are essential for surgeons performing sternal closure procedures. Inadequate surgical technique or lack of proficiency in sternal closure methods can increase the risk of adverse events and compromise patient outcomes. Continuous education and training programs help ensure that healthcare professionals possess the necessary skills and knowledge to perform sternal closure procedures safely and effectively.Key Market Trends
Increasing Demand for Minimally Invasive Procedures
Patients often prefer minimally invasive procedures due to perceived benefits such as smaller incisions, reduced post-operative pain, shorter recovery times, and improved cosmetic outcomes compared to traditional open surgeries. The desire for less invasive treatment options drives the demand for sternal closure systems compatible with minimally invasive approaches. Technological advancements and innovations in surgical techniques have expanded the scope of minimally invasive procedures across various medical specialties, including cardiac and thoracic surgery. Surgeons now have access to advanced instrumentation, imaging technologies, and surgical tools that enable them to perform complex procedures through smaller incisions with greater precision and efficiency. Minimally invasive procedures minimize tissue trauma and disruption compared to traditional open surgeries, leading to faster recovery times and reduced post-operative complications. Sternal closure systems designed for minimally invasive approaches help maintain sternal stability while facilitating optimal healing and recovery following cardiac and thoracic surgeries. Minimally invasive techniques are associated with improved patient outcomes, including lower rates of wound infections, reduced hospital stays, and faster return to normal activities. The adoption of sternal closure systems tailored to minimally invasive procedures contribute to enhanced patient satisfaction, reduced healthcare costs, and improved overall healthcare quality. Minimally invasive approaches are increasingly utilized for a wide range of cardiac and thoracic procedures, including coronary artery bypass grafting (CABG), valve repair or replacement, atrial fibrillation surgery, and thoracic surgeries. As surgeons gain experience and confidence in performing minimally invasive procedures, the demand for sternal closure systems compatible with these techniques continues to grow.Segmental Insights
Product Insights
The closure devices segment is projected to experience rapid growth in the Global Sternal Closure Systems Market during the forecast period. There is a growing trend towards minimally invasive cardiac surgeries and thoracic procedures, which require specialized closure devices suitable for smaller incisions and reduced tissue trauma. Closure devices designed for minimally invasive approaches offer benefits such as faster recovery times, shorter hospital stays, and improved cosmetic outcomes, driving their adoption in clinical practice. The rising incidence of cardiovascular diseases, including coronary artery disease, congestive heart failure, and valvular heart disease, contributes to the growing demand for cardiac surgeries that require sternal closure. As the prevalence of these conditions continues to increase, particularly among aging populations, the need for effective closure devices rises accordingly. Healthcare providers are increasingly prioritizing patient safety, surgical outcomes, and quality of care. Closure devices play a critical role in achieving secure sternal closure, minimizing the risk of complications such as sternal dehiscence and mediastinitis, and promoting optimal healing following cardiac and thoracic surgeries.Procedure Insights
The median sternotomy segment is projected to experience rapid growth in the Global Sternal Closure Systems Market during the forecast period. Median sternotomy, which involves a vertical incision through the sternum, is a common approach for many cardiac surgeries. As minimally invasive techniques continue to gain popularity, particularly for procedures like minimally invasive coronary artery bypass grafting (CABG) and valve surgeries, the demand for sternal closure systems suitable for median sternotomy procedures is expected to rise. Technological advancements have led to the development of new surgical techniques that allow for smaller incisions and reduced trauma to the sternum. These techniques often require specialized sternal closure systems designed to provide adequate stability and support while accommodating the unique requirements of minimally invasive procedures. Minimally invasive approaches offer several potential benefits to patients, including shorter recovery times, reduced post-operative pain, and improved cosmetic outcomes compared to traditional open surgeries. As healthcare providers prioritize patient-centered care and strive to optimize surgical outcomes, there is growing interest in adopting techniques that minimize surgical trauma and enhance patient recovery. The indications for median sternotomy procedures continue to expand beyond traditional cardiac surgeries to include procedures such as thoracic surgeries, aortic surgeries, and certain oncological interventions. This broader range of applications increases the demand for sternal closure systems capable of accommodating diverse surgical techniques and patient populations.Regional Insights
North America emerged as the dominant player in the Global Sternal Closure Systems Market in 2023. North America boasts a highly developed healthcare infrastructure with state-of-the-art medical facilities, advanced technologies, and skilled healthcare professionals. This enables the region to offer sophisticated cardiac surgical procedures, including sternal closure systems, with high standards of quality and safety. North America has a relatively high prevalence of cardiovascular diseases, including coronary artery disease, which necessitates a significant number of cardiac surgeries such as coronary artery bypass grafting (CABG). As a result, there is a substantial demand for sternal closure systems in the region to support these procedures. The region is a hub for medical device innovation, with numerous companies investing in research and development to enhance the effectiveness and safety of sternal closure systems. The continuous introduction of technologically advanced products contributes to the growth of the market in North America. North America has stringent regulatory standards and approval processes enforced by regulatory bodies such as the Food and Drug Administration (FDA) in the United States and Health Canada. While navigating these regulatory requirements can be challenging for manufacturers, they help ensure the safety and efficacy of sternal closure systems, thereby instilling confidence among healthcare providers and patients.Report Scope:
In this report, the Global Sternal Closure Systems Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Sternal Closure Systems Market, By Product:
- Closure Devices
- Bone Cement
Sternal Closure Systems Market, By Procedure:
- Median Sternotomy
- Hemisternotomy
- Bilateral Thoracosternotomy
Sternal Closure Systems Market, By Material:
- Stainless Steel
- PEEK
- Titanium
- Others
Sternal Closure Systems Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Sternal Closure Systems Market.Available Customizations:
Global Sternal Closure Systems market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Zimmer Biomet Holdings Inc.
- Medical Devices Business Services, Inc.
- KLS Martin Group
- Acute Innovations LLC
- IDEAR S.R.L
- A&E Medical Corporation
- Kinamed Incorporated
- JACE Medical, LLC
- B. Braun Melsungen AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | February 2024 |
| Forecast Period | 2023 - 2029 |
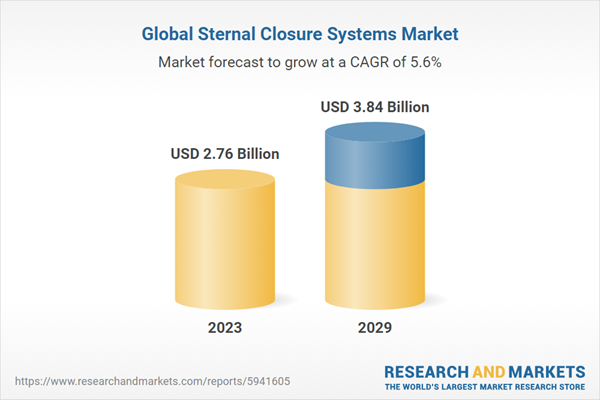
| Estimated Market Value ( USD | $ 2.76 Billion |
| Forecasted Market Value ( USD | $ 3.84 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |