Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Preterm Birth Rates
The rising preterm birth rate plays an important role in driving the growth of global Preterm Birth and PROM (premature rupture of membrane) testing Market. Preterm birth refers to as the delivery of baby before 37 weeks of gestation. By 2021, approximately 1 in 10 children born in the United States will be affected by premature birth. The preterm birth rate increased by 4 percentage points from 10.1% in 2020 to 10.5% in 2021. With the increased preterm birth rate globally, there is also growing demand for testing methods that can accurately predict and diagnose preterm labor and PROM. According to World Health Organization (WHO) In 2020, an estimated 13.4 million babies were born prematurely, and nearly 1 million died from premature complications. Healthcare providers and pregnant women seek reliable tests to identify these conditions early, allowing for appropriate interventions and management strategies. With the increased preterm birth rate there is also growth in the advancements in screening and diagnostic tests for preterm labor and PROM. These tests include fetal fibronectin (fFN) testing, cervical length measurement, transvaginal ultrasound, and other laboratory evaluations. The growing market demand drives the development and improvement of these tests, resulting in more accurate and efficient diagnostic capabilities. Preterm birth and PROM testing go hand in hand with monitoring and managing these conditions. Healthcare providers require tools and technologies for continuous monitoring of uterine activity, fetal well-being, and maternal health parameters. The rising preterm birth rate has spurred the development of monitoring solutions, such as electronic fetal monitoring systems, non-invasive monitoring devices, and telemedicine platforms, to facilitate early detection and effective management of preterm labor and PROM.Growing Awareness and Focus on Maternal Health & Child Health
Growing awareness and focus on maternal health and child health plays a significant role in driving the growth of Global Preterm Birth and PROM Testing Market. According to World Health Organization (WHO) Complications of premature birth are the leading cause of death for children under the age of 5, with nearly 900,000 deaths in 2019. With the increased awareness about the risks and consequences of preterm birth has urged governments, healthcare organizations, and societies to take active measures to reduce preterm birth rates. This includes executing programs and initiatives aimed at preventing preterm birth and improving neonatal outcomes. As a result, there is a greater demand for preterm birth and PROM testing to identify high-risk pregnancies and provide appropriate interventions with the increasing awareness more people were seeking for the effective testing which drives the growth of Global Preterm Birth and PROM Testing Market. With the increased focus on maternal and child health also led to a greater emphasis on early detection and intervention in cases of preterm labor and PROM. Early detection of these conditions makes it easy for healthcare providers to take necessary steps to prolong pregnancy or manage complications, enhancing the chances of a healthy outcome for both the mother and the baby. This drives the demand for preterm birth and PROM testing to facilitate early diagnosis and management and propels the growth of Global Preterm Birth and PROM Testing Market.Advancement in Technology for Testing
With the advancement in technology in testing it has become easy to diagnose PROM condition with more accuracy and correct data, this plays an important role in driving the growth of Global Preterm Birth and PROM Testing Market. Technological advancements have led to the development of more accurate and reliable testing methods for preterm birth and PROM. These advancements include improvements in imaging techniques, biomarker analysis, and genetic testing, among others. Enhanced accuracy and reliability in testing methods instill confidence in healthcare providers and patients, leading to increased adoption and demand for these tests. Technology has enabled the development of non-invasive testing options for preterm birth and PROM. Non-invasive tests, such as cervical length measurement using ultrasound or biochemical markers in maternal blood, offer a safer and more convenient alternative to invasive procedures. The availability of non-invasive testing methods increases patient acceptance and accessibility, driving market growth.Increased Healthcare Expenditure in Developing Nations
The global demand for Preterm Birth and Premature Rupture of Membranes (PROM) testing is experiencing a notable increase, propelled by the rising healthcare expenditure in developing nations. As developing countries allocate more resources to bolster their healthcare infrastructures, there is a growing focus on maternal and child health, including the early detection of complications related to preterm birth and PROM. Increased healthcare expenditure enables these nations to invest in advanced diagnostic technologies and screening programs, leading to a surge in demand for tests that can identify and manage the risks associated with preterm birth and PROM. Healthcare providers in developing nations are increasingly recognizing the importance of early detection in improving maternal and neonatal outcomes, driving the adoption of comprehensive testing protocols.Additionally, as awareness campaigns and education initiatives gain traction, both healthcare professionals and expectant mothers are seeking accessible and reliable testing solutions. The demand for Preterm Birth and PROM testing is poised to continue its upward trajectory, fostering collaborations between healthcare stakeholders, diagnostic manufacturers, and policymakers. This trend not only contributes to better healthcare outcomes in developing nations but also underscores the global significance of advancing diagnostic technologies that address critical maternal and neonatal health challenges.
Key Market Challenges
High Cost of Testing
The global demand for Preterm Birth and Premature Rupture of Membranes (PROM) testing is facing a decline attributed to the high cost associated with these diagnostic procedures. The sophisticated nature of testing technologies, coupled with the need for specialized equipment and expertise, contributes to elevated testing costs. This financial barrier poses challenges to accessibility, particularly in regions where healthcare resources are limited, leading to a decreased demand for Preterm Birth and PROM testing on a global scale. The economic considerations for both healthcare providers and individuals play a pivotal role in the decision-making process, affecting the widespread adoption of these essential diagnostic services. The high cost of testing not only impacts the ability of healthcare systems to implement comprehensive screening programs but also influences the choices made by expectant mothers seeking prenatal care.Lack of Trained Professionals
The global demand for Preterm Birth and Premature Rupture of Membranes (PROM) testing is experiencing a downturn due to a pervasive challenge the shortage of trained professionals in the healthcare sector. Performing accurate and reliable testing for preterm birth and PROM requires specialized skills and knowledge. However, there is a notable scarcity of healthcare professionals proficient in conducting these tests, particularly in regions facing workforce shortages or limited access to training programs. The lack of trained professionals hampers the efficient implementation of comprehensive prenatal testing programs, leading to a decreased demand for these essential diagnostics on a global scale. The intricacies of these tests necessitate a skilled workforce to ensure accurate results and proper interpretation.Key Market Trends
Increased Screenings Due to Rising Maternal Age
The global demand for Preterm Birth and Premature Rupture of Membranes (PROM) testing is experiencing a significant uptick, driven by the increasing trend of delayed maternal age worldwide. As more women choose to conceive at an older age, healthcare providers are witnessing a heightened awareness of the potential risks associated with advanced maternal age, including an increased likelihood of preterm birth and PROM. In response to this demographic shift, there has been a surge in demand for comprehensive testing protocols to assess and manage these risks effectively. Increased screenings, which have become a standard part of prenatal care for older mothers, contribute to the growing demand for advanced diagnostic solutions.Expectant mothers, in collaboration with healthcare professionals, are seeking reliable testing options to proactively address and mitigate potential complications. The rise in maternal age underscores the need for accessible and accurate Preterm Birth and PROM testing globally. Diagnostic manufacturers and healthcare stakeholders are responding to this demand by innovating and expanding their offerings to meet the specific needs of an older maternal population. This evolving landscape not only reflects a changing demographic trend but also emphasizes the crucial role of advanced diagnostic technologies in ensuring the health and well-being of both mothers and newborns on a global scale.
Governmental Policies Encouraging Prenatal Testing
The global demand for Preterm Birth and Premature Rupture of Membranes (PROM) testing is on the rise, driven by governmental policies worldwide that encourage and prioritize prenatal testing initiatives. Recognizing the importance of early detection and management of pregnancy-related complications, many governments have implemented policies advocating for comprehensive prenatal testing, including screenings for preterm birth and PROM. These initiatives aim to improve maternal and neonatal health outcomes by fostering a proactive approach to identifying potential risks during pregnancy. As a result, there has been a considerable increase in the demand for testing solutions that can provide accurate and timely information about preterm birth and PROM risks.Governmental support and funding for prenatal testing programs contribute to heightened awareness among healthcare providers and expectant mothers, further fueling the demand for these advanced diagnostics globally. The emphasis on preventive healthcare measures aligns with the broader goal of reducing maternal and neonatal morbidity and mortality rates. Diagnostic manufacturers and healthcare stakeholders are responding to this increased demand by developing and offering innovative testing solutions that adhere to regulatory standards and government recommendations. The intersection of governmental policies and advancements in prenatal testing technologies is reshaping the landscape of maternal healthcare, fostering a global environment where the importance of early detection is prioritized and supported by public health initiatives.
Segmental Insights
Test Type Insights
Based on the Test Type, in the ever-evolving landscape of the Global Preterm Birth and PROM Testing Market, the utilization of Biochemical markers, particularly Fetal Fibronectin (fFN), has emerged as the dominant approach. This testing method has garnered widespread adaptation and preference due to its remarkable attributes. Notably, it exhibits high sensitivity and specificity in predicting preterm labor, ensuring accurate and reliable results. Moreover, it is considered less invasive and more comfortable for patients when compared to alternative methods such as Pelvic Exam or Uterine Monitoring. However, it's important to acknowledge the dynamic nature of the market. With continuous advancements and breakthroughs in medical technology, the market dynamics may undergo transformation over time. As new innovations emerge, they have the potential to shape and redefine the landscape of preterm birth and PROM testing, offering even more precise and efficient diagnostic approaches.Application Insights
Based on the Application segment, among PROM (Premature Rupture of Membranes), Preterm Labor, and Chorioamnionitis, it is the PROM condition that is currently dominating the Global Preterm Birth and PROM Testing Market. This dominance can be attributed to the rising prevalence of PROM, a condition characterized by the premature rupture of the amniotic sac, and the subsequent need for early detection to mitigate potential complications for both the mother and the baby. By detecting PROM early, healthcare providers can intervene promptly and implement appropriate management strategies to ensure the well-being of both mother and baby, reducing the risk of adverse outcomes associated with this condition. The need for accurate and reliable PROM testing methods has become increasingly paramount in the field of obstetrics, as healthcare professionals strive to improve pregnancy outcomes and minimize the potential risks associated with preterm birth.Regional Insights
In the Global Preterm Birth and PROM Testing Market, North America is currently dominating with its advanced healthcare infrastructure, higher awareness about the need for early testing, and the presence of key market players in the region. The well-established healthcare facilities and research institutions in North America contribute to the region's leadership in this market. Moreover, North America's strong collaboration between healthcare professionals and industry experts further strengthens the development and adoption of innovative testing solutions. Through continuous research and technological advancements, healthcare professionals in North America are continuously striving to improve the accuracy and efficiency of preterm birth and PROM testing.Furthermore, the region's focus on preventive care and early detection has played a pivotal role in reducing the risks associated with preterm birth and PROM. By implementing comprehensive screening programs and promoting awareness among the general population, North America has made significant progress in addressing the challenges related to these conditions. With a proactive approach and a commitment to improving outcomes, North America continues to lead the way in the field of preterm birth and PROM testing. The region's dedication to research, innovation, and collaboration sets a strong foundation for the development of novel strategies and interventions that aim to enhance the overall health and well-being of mothers and infants worldwide.
Report Scope:
In this report, the Global Preterm Birth and PROM Testing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Preterm Birth And PROM Testing Market, By Test Type:
- Pelvic Exam
- Ultrasound
- Biochemical Markers
- Uterine Monitoring
- Nitazine Test
- Ferning Test
- Pooling
- PAMG-1 Immunoassay
- IGFBP Test
- Fetal Fibronectin
- Others
Preterm Birth And PROM Testing Market, By Application:
- PROM
- Preterm Labor
- Chorioamnionitis
Preterm Birth And PROM Testing Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Preterm Birth and PROM Testing Market.Available Customizations:
Global Preterm Birth and PROM Testing Market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Qiagen N.V.
- Hologic, Inc.
- Cooper Surgical Inc.
- Abbott Laboratories Inc.
- Medix Biochemica Oy AB
- Sera Prognostics Inc.
- Clinical Innovations, LLC
- Biosynex
- NX Prenatal, Inc.
- IQ Products Co.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | February 2024 |
| Forecast Period | 2023 - 2029 |
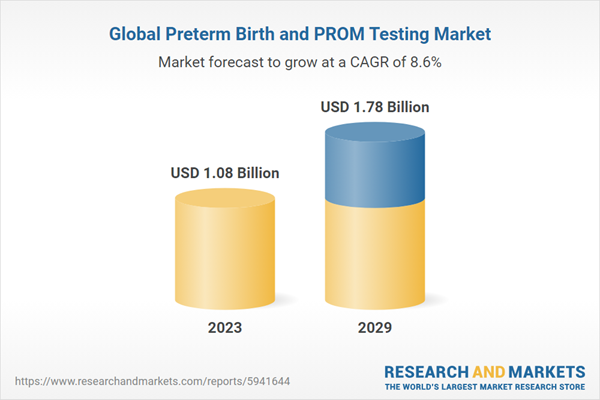
| Estimated Market Value ( USD | $ 1.08 Billion |
| Forecasted Market Value ( USD | $ 1.78 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |