Reagents & Kits is the fastest growing segment, North America is the largest market globally
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The expanding pipeline of cell and gene therapies and stringent global regulatory requirements for biologics represent two pivotal drivers for the Global Biological Safety Testing Products and Services Market. The increasing proliferation of novel advanced therapeutic modalities, particularly cell and gene therapies, inherently demands extensive and specialized safety assessments throughout their development and manufacturing.These therapies involve complex biological constructs that necessitate rigorous testing to ensure product purity, potency, and freedom from adventitious agents. According to the Alliance for Regenerative Medicine's annual Cell & Gene State of the Industry Briefing, in January 2024, seven cell and gene therapies received FDA approval in the U. S. in 2023, with predictions for up to 17 approvals in the U.S. and EU in 2024.
Key Market Challenges
The substantial capital expenditure required for establishing and maintaining advanced testing infrastructure presents a significant impediment to the growth of the Global Biological Safety Testing Products and Services Market. This financial barrier limits the ability of new service providers to enter the market and hinders existing firms from expanding their capabilities or upgrading to the latest technologies. Developing and equipping laboratories with advanced assays, reagents, and instruments for complex biological product analysis demands considerable upfront investment, directly constraining the overall capacity available to meet increasing industry demand for safety assessments.Key Market Trends
The Global Biological Safety Testing Products and Services Market is significantly shaped by advancements in rapid and high-throughput testing technologies. These innovations address the critical industry need for accelerated development timelines and increased efficiency in assessing biological product safety. The integration of advanced platforms, such as next-generation sequencing and automated systems, allows for faster detection of contaminants and more comprehensive characterization of complex biologics.Key Market Players Profiled:
- Charles River Laboratories International, Inc.
- BSL Bioservice Scientific Laboratories Munich GmbH
- Merck KGaA
- Samsung Biologics
- Sartorius AG
- Eurofins Scientific (Ireland) Limited
- SGS Société Générale de Surveillance SA
- Thermo Fisher Scientific Inc.
- BIOMÉRIEUX SA
- Lonza Group Ltd.
Report Scope:
In this report, the Global Biological Safety Testing Products and Services Market has been segmented into the following categories:By Product:
- Reagents & Kits
- Services
- Instruments
By Application:
- Vaccines & Therapeutics
- Gene Therapy
- Monoclonal Antibodies
- Recombinant Protein
- Blood & Blood-based Products
- Tissue & Tissue-based Products
- Stem Cells
By Test Type:
- Endotoxin Tests
- Sterility Tests
- Cell Line Authentication & Characterization Tests
- Bioburden Tests
- Adventitious Agent Detection Tests
- Residual Host Contamination Detection Tests
- Others
By Region:
- North America
- Europe
- Asia Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Biological Safety Testing Products and Services Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Charles River Laboratories International, Inc.
- BSL Bioservice Scientific Laboratories Munich GmbH
- Merck KGaA
- Samsung Biologics
- Sartorius AG
- Eurofins Scientific (Ireland) Limited
- SGS Société Générale de Surveillance SA
- Thermo Fisher Scientific Inc.
- BIOMÉRIEUX SA
- Lonza Group Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | November 2025 |
| Forecast Period | 2024 - 2030 |
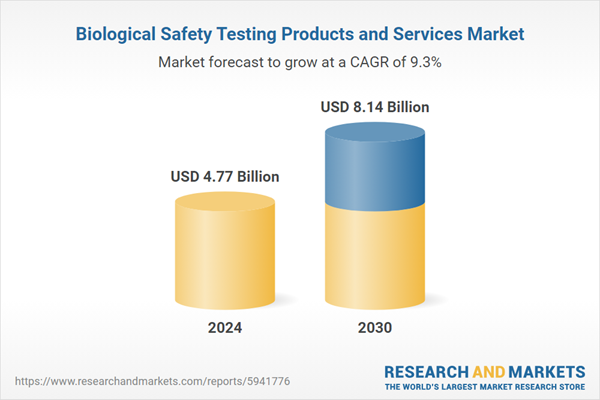
| Estimated Market Value ( USD | $ 4.77 Billion |
| Forecasted Market Value ( USD | $ 8.14 Billion |
| Compound Annual Growth Rate | 9.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |