Oral is the fastest growing segment, North America is the largest market globally
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The Global Antiviral Combination Therapy Market is significantly influenced by the persistently rising prevalence of viral infections worldwide. The continuous emergence and spread of diverse viral pathogens necessitate advanced and often multi-drug treatment approaches to manage disease progression effectively and mitigate severe outcomes. For instance, according to the Pan American Health Organization / World Health Organization, in December 2023, the Region of the Americas alone reported over 4.2 million new cases of dengue fever, surpassing the previous record from 2019 by more than a million cases, as highlighted in their Situation Report N.1: Dengue Epidemiological Situation in the Americas. Such widespread outbreaks and increasing incidence rates across various viral diseases create an urgent and sustained demand for robust antiviral combination therapies that can address complex viral dynamics and patient needs.Key Market Challenges
The high cost associated with developing novel combination therapies and their subsequent pricing presents a significant impediment to the growth of the Global Antiviral Combination Therapy Market. The substantial financial investment required for research and development directly translates into elevated drug prices, creating access barriers in numerous regions globally. This restricts patient access and limits the adoption of these advanced treatments, particularly in healthcare systems with budget constraints.Key Market Trends
The application of Artificial Intelligence in drug discovery represents a significant trend in the global antiviral combination therapy market by accelerating the identification and optimization of novel therapeutic agents. AI algorithms are increasingly deployed to analyze complex biological data, predict drug efficacy, and streamline the screening of potential antiviral compounds, thereby reducing the time and cost associated with early-stage development. This technological integration is evidenced by the active engagement of international organizations, with the Council for International Organizations of Medical Sciences (CIOMS) holding meetings for its Working Group on Artificial Intelligence in Pharmacovigilance as recently as September 2025, signaling the industry's growing commitment to AI adoption across the pharmaceutical lifecycle.Key Market Players Profiled:
- Celltrion Inc.
- GlaxoSmithKline plc
- Gilead Sciences, Inc
- AbbVie, Inc.
- Bristol-Myers Squibb Company
- Janssen Pharmaceuticals, Inc.
- Cipla Limited
- Viatris Inc
- Merck & Co., Inc.
- F. Hoffmann-La Roche Ltd
Report Scope:
In this report, the Global Antiviral Combination Therapy Market has been segmented into the following categories:By Type:
- Branded
- Generic
By Drug Combination:
- DNA Polymerase Inhibitors
- Reverse Transcriptase Inhibitors
- Protease Inhibitors
- Neuraminidase Inhibitors
- Others
By Route of Administration:
- Oral
- Intravenous
By Indication:
- Human Immunodeficiency Virus
- Hepatitis
- Others
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Others
By Region:
- North America
- Europe
- Asia Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Antiviral Combination Therapy Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Celltrion Inc.
- GlaxoSmithKline plc
- Gilead Sciences, Inc
- AbbVie, Inc.
- Bristol-Myers Squibb Company
- Janssen Pharmaceuticals, Inc.
- Cipla Limited
- Viatris Inc
- Merck & Co., Inc.
- F. Hoffmann-La Roche Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | November 2025 |
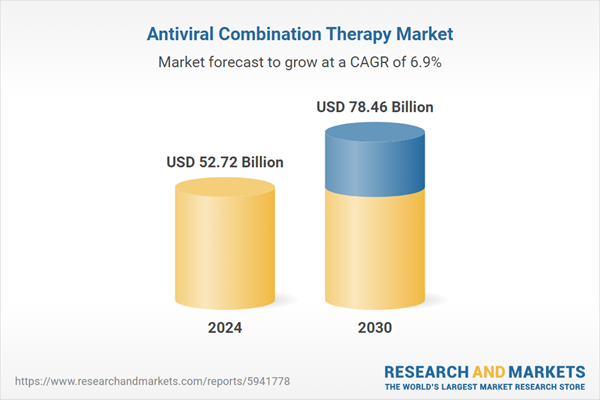
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 52.72 Billion |
| Forecasted Market Value ( USD | $ 78.46 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |