1 Oncology Molecular Diagnostic Market
1.1 Overview
1.2 Cancer Type
1.3 Technology
1.4 Market Dynamics
1.5 Regulatory-Approved Products
2 Next-Generation Breast Cancer Diagnostic and Screening Market
2.1 Market Outlook
2.1.1 Product Definition
2.1.2 Definition by Technologies Involved
2.1.2.1 Real-Time Polymerase Chain Reaction (RT-PCR)
2.1.2.2 Fluorescence In-Situ Hybridization (FISH)
2.1.2.3 Next-Generation Sequencing
2.1.2.4 Immunohistochemistry (IHC)
2.1.3 Inclusion and Exclusion
2.1.4 Overview
2.1.4.1 Market Size and Growth Potential, $Million, 2022-2032
3 Industry Outlook
3.1 Key Trends
3.1.1 Liquid Biopsy for Breast Cancer Diagnosis
3.1.2 Complete Automation of Next-Generation Techniques
3.2 Regulatory Framework
3.2.1 Regulatory Framework in the U.S.
3.2.1.1 FDA Regulation
3.2.1.2 CMS Regulations (Reimbursement Scenario)
3.2.2 Regulatory Framework in Europe
3.2.3 Regulatory Framework in Asia-Pacific
3.2.3.1 China
3.2.3.2 Japan
3.3 Regulatory-Approved Products
3.4 Clinical Trials
3.5 Breast Cancer Incidence Growth Rates (by Country)
3.6 Advantages and Limitations of Different Techniques
3.7 Pricing
4 Regions
4.1 Asia-Pacific
4.1.1 Key Findings
4.1.2 Market Dynamics
4.1.2.1 Impact Analysis
4.1.3 Market Sizing and Forecast
4.1.3.1 Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Country)
4.1.3.1.1 China
4.1.3.1.1.1 Market Dynamics
4.1.3.1.1.2 Market Sizing and Forecast
4.1.3.1.2 Japan
4.1.3.1.2.1 Market Dynamics
4.1.3.1.2.2 Market Sizing and Forecast
4.1.3.1.3 India
4.1.3.1.3.1 Market Dynamics
4.1.3.1.3.2 Market Sizing and Forecast
4.1.3.1.4 Australia
4.1.3.1.4.1 Market Dynamics
4.1.3.1.4.2 Market Sizing and Forecast
4.1.3.1.5 South Korea
4.1.3.1.5.1 Market Dynamics
4.1.3.1.5.2 Market Sizing and Forecast
4.1.3.1.6 Singapore
4.1.3.1.6.1 Market Dynamics
4.1.3.1.6.2 Market Sizing and Forecast
4.1.3.1.7 Rest-of-Asia-Pacific
4.1.3.1.7.1 Market Dynamics
4.1.3.1.7.2 Market Sizing and Forecast
5 Markets - Competitive Benchmarking
5.1 Next-Generation Breast Cancer Diagnostic and Screening Active Players Ecosystem
5.2 Company Profiles
5.2.1 BGI Genomics Co., Ltd.
5.2.1.1 Company Overview
5.2.1.2 Role of BGI Genomics Co., Ltd. in the Next-Generation Breast Cancer Diagnostic and Screening Market
5.2.1.3 Financials
5.2.1.4 Recent Developments
5.2.1.5 Analyst Perception
List of Figures
Figure 1: Worldwide Breast Cancer Statistics (by Region), 2020
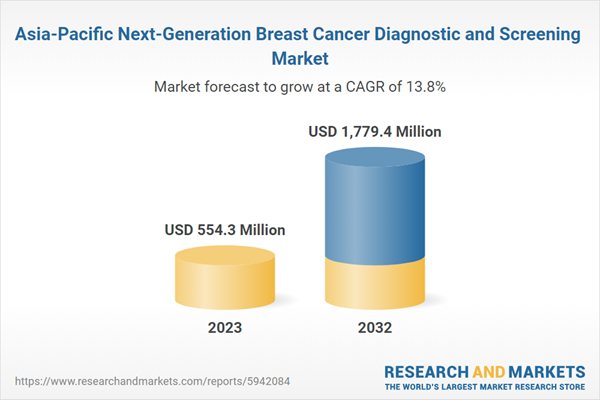
Figure 2: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 3: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Technology), $Million, 2022 and 2032
Figure 4: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Biomarker), $Million, 2022 and 2032
Figure 5: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Cancer Sub-Type), $Million, 2022 and 2032
Figure 6: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Offering), $Million, 2022 and 2032
Figure 7: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by End User), $Million, 2022 and 2032
Figure 8: Next-Generation Breast Cancer Diagnostic and Screening Market, Impact Analysis
Figure 9: Next-Generation Breast Cancer Diagnostic and Screening Market, Key Development Analysis, January 2019-April 2023
Figure 10: Next-Generation Breast Cancer Diagnostic and Screening Market: Research Methodology
Figure 11: Primary Research Methodology
Figure 12: Bottom-Up Approach (Segment-Wise Analysis)
Figure 13: Top-Down Approach (Segment-Wise Analysis)
Figure 14: Oncology Molecular Diagnostic Market, Solid Tumor, $Million, 2022 and 2032
Figure 15: Oncology Molecular Diagnostic Market, Hematological Malignancy, $Million, 2022 and 2032
Figure 16: Oncology Molecular Diagnostic Market, Market Dynamics
Figure 17: Next-Generation Breast Cancer Diagnostic and Screening Market, Size and Growth Potential, $Million, 2022-2032
Figure 18: Market Size and Growth Potential (by Region), $Million, 2022-2032
Figure 19: FDA Guidelines for CDx Approval
Figure 20: Criteria for CMS Coverage/Reimbursement
Figure 21: Europe In-Vitro Diagnostic Devices Regulation Regulatory Process
Figure 22: Breast Cancer Incidence Growth Rates (by Country), 2020 and 2040
Figure 23: Advantages and Limitations of Different Techniques in the Next-Generation Breast Cancer Diagnostic and Screening Market
Figure 24: Next-Generation Breast Cancer Diagnostic and Screening Market Share (by Region), 2022-2032
Figure 25: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market, Incremental Opportunity (by Country), $Million, 2023-2032
Figure 26: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 27: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market (by Country), Share (%), 2022 and 2032
Figure 28: China Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 29: Japan Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 30: India Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 31: Australia Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 32: South Korea Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 33: Singapore Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 34: Rest-of-Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market, $Million, 2022-2032
Figure 35: BGI Genomics Co., Ltd. Product Portfolio
Figure 36: BGI Genomics Co., Ltd.: Overall Financials, $Million, 2020-2022
List of Tables
Table 1: Key Questions Answered in the Report
Table 2: Some of the Regulatory-Approved Oncology Molecular Diagnostic Products/Services for Different Types of Solid Tumors
Table 3: Some of the Regulatory-Approved Next-Generation Breast Cancer Diagnostic and Screening Products/Tests
Table 4: Next-Generation Breast Cancer Diagnostic and Screening, Clinical Trials
Table 5: Pricing of Some Products/Services for Next-Generation Breast Cancer Diagnostic and Screening
Table 6: Asia-Pacific Next-Generation Breast Cancer Diagnostic and Screening Market, Impact Analysis
Table 7: Next-Generation Breast Cancer Diagnostic and Screening Active Players Ecosystem
Table 8: BGI Genomics Co. Ltd.: Key Products and Features