Smart pills, also referred to as digital pills or ingestible sensors, are innovative medical technologies aimed at facilitating better medicine adherence and health monitoring. These small, swallowable devices contain sensors that can communicate with wearable devices or smartphones, instantly passing on crucial information to both the patient and healthcare professional. The primary objective of smart pills is to increase the accuracy of medication tracking and ensure that patients take their prescribed medications on time.
Smart pills have gained popularity due to the increasing prevalence of chronic diseases that require effective management solutions across the globe. This technology will largely benefit patients with diabetes, cardiovascular diseases, and mental health disorders by improving medication adherence and thus benefiting their overall health outcomes.
Further, the integration of smart pills with telemedicine and digital health has made them even more attractive. As health systems around the world continue to move toward personalized medicine and evidence-based practices, smart pills will be critical to medication compliance and overall quality patient care. This technology empowers not just patients but also providers of healthcare, making it a game-changer in modern medicine.
Top 5 Companies in the Global Smart Pills Market
General Electric Company
Establishment: 1892Headquarters: United States of America
General Electric Co (GE) is an industrial conglomerate. It provides a range of industrial, infrastructure and financial products and services. The company offers a wide range of products and services from aircraft engines and systems, healthcare systems and pharmaceutical diagnostics, power generation, and oil and gas production equipment to medical imaging, financing and industrial products. It also offers a comprehensive range of financial services to its customers. The company serves water, oil and gas, power, energy management, aviation, healthcare, digital, transportation, appliances and lighting industries, among others. It has operations in North America, Europe, Asia, the Middle East, and Africa and other regions; and has manufacturing facilities across the world. GE is headquartered in Boston, Massachusetts, the US.
Nihon Kohden Corporation
Establishment: 1951Headquarters: Japan
Nihon Kohden Corp (Nihon Kohden) develops, manufactures and distributes medical electronic equipment. Nihon Kohden's major products include a variety of medical devices such as electroencephalographs, electrocardiographs, patient monitoring systems, defibrillators, and in-vitro diagnostic equipment. The company's products are used in various clinical areas from the emergency response to testing, diagnosis, treatment, and rehabilitation, as well as in health improvement, home medical care, nursing, and basic medical research. The company's products are distributed internationally: the company exports its medical equipment and devices to other countries all over the world to satisfy local medical demands. The company also imports a range of products from abroad into Japan; the areas include cardiology, urology, respiratory care, anesthesiology, emergency care, sports medicine, and rehabilitation.
Hill-Rom Holdings, Inc.
Establishment: 1915Headquarters: United States of America
Hill-Rom Holdings Inc (Hillrom), a subsidiary of Baxter International Inc, is a medical technology company that provides patient support systems, surgical solutions, and front-line care products. Its major products and services include patient mobility solutions, specialty frames and surfaces, non-invasive therapeutic products and surfaces, patient monitoring and diagnostics products, respiratory health products, and surgical products that are used within the operating room setting. The company offers its products for acute care hospitals, extended care facilities, and home healthcare agencies. The company is also enhancing its clinical programs that focus on providing tools for building a safe environment for patients and staff, measurement and treatment of pressure injuries, and fall prevention. The company distributes its products in the US and international markets through its wholly owned subsidiaries, direct sales force, and a network of distributors.
OSI Systems, Inc.
Establishment: 1987Headquarters: United States of America
OSI Systems Inc (OSI Systems) is a vertically integrated manufacturer and designer of electronic systems and components. The company's product portfolio includes turnkey security screening solutions, patient monitoring systems, security and inspection systems, flex circuits, optoelectronic devices, diagnostic cardiology systems, connected care systems, and associated accessories. It also offers electronic manufacturing services, before and after product sales and technical support services. The company caters to the homeland security, government, healthcare, aerospace, OEM, and defense industries. OSI Systems operates through regional direct sales, marketing staff, and a network of independent distributors. The company operates in the Americas, Europe, the Middle East, and Asia-Pacific.
Boston Scientific Corporation
Establishment: 1979Headquarters: United States of America
Boston Scientific Corp (Boston Scientific) is a medical technology company that develops, manufactures and commercializes devices for a range of interventional medical specialties. The company provides products in the areas of electrophysiology, gastroenterology, gastrointestinal surgery, female pelvic medicine, gynecology, interventional cardiology, interventional radiology, neurological surgery, orthopedic surgery, pain medicine, pulmonology, urology, and vascular surgery. Boston Scientific supplies its products to hospitals, clinics, outpatient facilities, and medical offices worldwide. The company has manufacturing facilities in the US, Ireland, Costa Rica, Brazil, Malaysia, and Puerto Rico. It markets products directly and through a network of distributors and dealers in Europe, the Middle East, Africa, Asia Pacific, and the Americas.
Product Launches in the Global Smart Pills Market
CapsoVision, Inc.
In January 2025, the US-based CapsoVision, Inc. received FDA clearance for CapsoCam Plus for use in pediatric patients aged two and older. This milestone enables children to benefit from the ease and precision of capsule endoscopy, offering a non-invasive, comfortable diagnostic option that minimizes the stress typically associated with traditional endoscopy procedures.AnX Robotics
In January 2024, AnX Robotics (US) received FDA clearance for expanded indications for NaviCam Small Bowel Video Capsule Endoscopy (SB) for both adults and children aged 2 years and older. This milestone, combined with the recent FDA clearance of ProScan, the groundbreaking AI-assisted reading tool for Small Bowel Video Capsule Endoscopy (VCE), cements NaviCam SB as the most advanced technology in small bowel video capsule endoscopy.SWOT Analysis of Company
Koninklijke Philips N.V.
Strength: Leading Competency in Advanced Digital Health Integration and Connected CareThe biggest strengths that Koninklijke Philips N.V. possesses in the smart pills market are its advanced digital health ecosystem and leadership of connected medical technologies. With in-depth experience in the integration of sensor-based diagnostics, remote monitoring, and data analytics, Philips remains one of the leading players in smart pill innovation. Its experience with the development of miniaturized medical devices and wireless communication platforms enables seamless data transmission between the ingestible sensors and healthcare systems. The company has strong R&D capabilities and strategic partnerships with hospitals and technology companies for continuous innovation within personalized medicine, gastrointestinal monitoring, and patient compliance solutions. Additionally, Philips' quality reputation across global markets and a strategy focused on AI-powered healthcare platforms further strengthen its competitive advantage in the market for smart medical devices. Philips combines digital intelligence with clinical reliability and patient-centered design to lead the evolution of smart pills as part of the broader revolution in connected healthcare.
Medtronic plc
Strength - Strong Medical Device Expertise and Innovation in Miniaturized Therapeutics
The biggest strength for Medtronic plc in the smart pills market is a deep competency in medical device engineering and miniaturized therapeutic technologies. Having developed numerous implantable and sensor-based devices that combine advanced electronics, biocompatible materials, and real-time data transmission over many decades, Medtronic is well-placed to develop sophisticated smart pills that meet a wide range of clinical needs in drug delivery, diagnostic monitoring, and gastrointestinal applications. Strong R&D investments by Medtronic and strategic partnerships with pharmaceutical and digital health companies foster continuous innovation in targeted drug delivery and precision medicine. With established global regulatory experience and strong clinical trial infrastructure, the company ensures rapid commercialization of safe and effective devices. Backed by a well-trusted brand and large distribution network, Medtronic emerges as one of the leading players in shaping the future of smart pill technology and connected therapeutics with its technological excellence, clinical expertise, and digital integration.Recent Development in the Global Smart Pills Market
Olympus Corporation
September 2025 - Olympus Corporation, a global MedTech company focused on improving people's lives, is pleased to announce the commercial launch of its OLYSENSE™ CAD/AI portfolio, powered by artificial intelligence, in the United States and European markets. This breakthrough portfolio harnesses the power of AI for earlier detection, increased diagnostic confidence, and improved patient outcomes. In Europe, the launch will include the clinical applications CADDIE™, CADU™, and SMARTIBD™. In the United States, the launch includes CADDIE™ with detection capabilities only.DetectRx’s
APRIL 2022, Belgium-based AARDEX Group, the global leader in medication adherence for clinical trials, announces its partnership with etectRx, Inc. The combination of etectRx's ID-Cap™ System with its ingestible wireless sensor and the MEMS Adherence Software from AARDEX Group provides a complete solution to monitor medication-taking behavior. With their combined solution, they provide a highly accurate method of measuring adherence to medications supplied in solid dosage form. Integration means the data captured can be analyzed by MEMS AS to help healthcare teams understand patient dosing patterns and introduce targeted interventions to optimize medication adherence.Sustainability Goal
IntroMedic Co. Ltd.
IntroMedic Co. Ltd. aims to bring a healthy and sustainable future by introducing innovative medical technology and ecological operations. As a capsule endoscopy and smart medical device leader, the company is devoted to minimizing the environmental impact of its activities while promoting better accessibility in healthcare. IntroMedic promotes energy efficiency, minimal waste generation, and the use of eco-friendly materials in all processes of manufacturing and packaging. Furthermore, it seeks to design products with a longer lifespan for recycling and reusing resources, which is in line with the principles of the global circular economy. On the social side, IntroMedic cares about ethical business practice, patient safety, and equality in accessing advanced diagnostic technologies. The company is committed to joining various studies related to early disease detection to help improve global health with minimal ecological harm. Therefore, through responsible innovation, sustainable manufacturing, and community-oriented values, IntroMedic aspires to balance technological advancement with care for the environment, proving that medical progress could go hand in hand with the sustainability of the planet.Fujifilm Holdings Corporation
Fujifilm Holdings Corporation's commitment to sustainability focuses on its corporate vision of creating a sustainable society through innovation in healthcare, the environment, and daily life. Under the guidance of the long-term plan "Sustainable Value Plan 2030," Fujifilm aspires to net-zero carbon emissions by 2040 and actively promotes circular economy initiatives. The company focuses on lessening environmental impact across the value chain through the use of renewable energy, the creation of recyclable packaging, and decreases in water consumption. Through its healthcare, imaging, and materials segments, Fujifilm makes sure that product design integrates sustainability into the manufacture of environmentally friendly medical devices, digital technologies, and green chemistry solutions. Emphasizing health equity, community well-being, and diversity within the global workforce are all specific examples of key social issues that Fujifilm focuses on. The company drives innovation that addresses global challenges such as the prevention of diseases and climate change. Aligning profitability with purpose, Fujifilm exemplifies how technological leadership and sustainability can play to each other's strengths in shaping a more healthy and resilient world.Market Segmentation
Smart Pills Market
- Historical Trends
- Forecast Analysis
Market Share Analysis - Smart Pills Market
General Electric Company
Overview
- Company History and Mission
- Business Model and Operations
Workforce
Key Persons
- Executive Leadership
- Operational Management
- Division Leaders
- Board Composition
Recent Development & Strategies
- Mergers & Acquisitions
- Partnerships
- Investments
Sustainability Analysis
- Renewable Energy Adoption
- Energy-Efficient Infrastructure
- Use of Sustainable Packaging Materials
- Water Usage and Conservation Strategies
- Waste Management and Circular Economy Initiatives
Product Analysis
- Product Profile
- Quality Standards
- Product Pipeline
- Product Benchmarking
Strategic Assessment: SWOT Analysis
- Strengths
- Weaknesses
- Opportunities
- Threats
Revenue Analysis
The above information will be available for all the following companies:
- General Electric Company
- Nihon Kohden Corporation
- Hill-Rom Holdings, Inc.
- OSI Systems, Inc.
- Boston Scientific Corporation
- Koninklijke Philips N.V.
- Medtronic plc
- Irhythm Technologies Inc
- Novartis International AG
- Olympus Corporation
- CapsoVision, Inc.
- AnX Robotics
- EtectRx, Inc.
- IntroMedic Co. Ltd.
- Check-Cap Ltd.
- BodyCap (France)
- Jinshan Science & Technology (Group) Co. Ltd.
- Otsuka Holdings Co., Ltd.
- Fujifilm Holdings Corporation
- Proteus Digital Health
Table of Contents
Companies Mentioned
- General Electric Company
- Nihon Kohden Corporation
- Hill-Rom Holdings, Inc.
- OSI Systems, Inc.
- Boston Scientific Corporation
- Koninklijke Philips N.V.
- Medtronic plc
- Irhythm Technologies Inc
- Novartis International AG
- Olympus Corporation
- CapsoVision, Inc.
- AnX Robotics
- EtectRx, Inc.
- IntroMedic Co. Ltd.
- Check-Cap Ltd.
- BodyCap (France)
- Jinshan Science & Technology (Group) Co. Ltd.
- Otsuka Holdings Co., Ltd.
- Fujifilm Holdings Corporation
- Proteus Digital Health
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | December 2025 |
| Forecast Period | 2025 - 2033 |
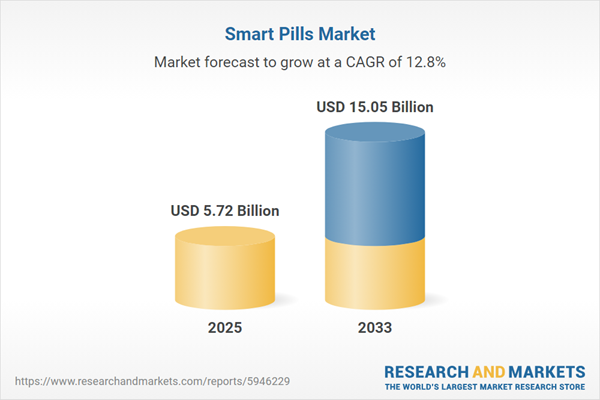
| Estimated Market Value ( USD | $ 5.72 Billion |
| Forecasted Market Value ( USD | $ 15.05 Billion |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |