Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these growth factors, the sector encounters a major obstacle due to the high attrition rate of drug candidates during clinical trials. The complex biology of the disease frequently results in scientific failures that delay regulatory approval and escalate development costs. This trend of clinical setbacks creates a precarious landscape for pharmaceutical developers and investors, significantly hindering the rapid expansion and commercialization of innovative therapeutic solutions in the global market.
Market Drivers
The expanding global geriatric population and the resulting rise in disease prevalence act as the primary catalysts for the growth of the Alzheimer’s therapeutics sector. As life expectancy increases worldwide, the incidence of neurodegenerative conditions is surging, generating an urgent and sustained demand for effective pharmaceutical interventions.This demographic shift exerts immense pressure on healthcare systems to adopt treatments capable of managing symptoms or slowing disease progression. According to a March 2024 'Dementia' fact sheet by the World Health Organization, there are currently over 55 million people living with dementia globally, with nearly 10 million new cases arising annually. This rapidly growing patient base requires the widespread availability of therapeutic agents, ensuring continuous revenue for developers and driving the strategic prioritization of dementia care in global health policies.
Simultaneously, the market is significantly influenced by the commercialization of new disease-modifying therapies and the broadening of the clinical pipeline. Pharmaceutical companies are increasingly shifting focus from symptomatic relief to targeting underlying disease mechanisms, such as amyloid-beta plaques, which fosters renewed investor confidence and drives product launches. This innovation trajectory is heavily supported by public sector investment designed to accelerate discovery; for instance, the National Institute on Aging reported that 2024 federal appropriations provided a $100 million increase for Alzheimer’s and related dementias research. The scale of this research is extensive, with the Alzheimer's Association's May 2024 report noting 164 agents currently in clinical trials, indicating a robust future for therapeutic options.
Market Challenges
The high attrition rate of drug candidates during clinical trials serves as a significant barrier to the growth of the global Alzheimer’s therapeutics market. This challenge arises largely from the complex biological nature of the disease, which often results in unexpected efficacy failures as pharmaceutical agents move from preclinical models to human testing. When promising candidates fail in late-stage trials, developers suffer massive financial losses and face extended development timelines. This recurring pattern creates a volatile environment that discourages sustained capital investment and compels pharmaceutical companies to adopt highly conservative strategies, ultimately slowing the pace of innovation and the market entry of new products.The direct result of these clinical setbacks is a restricted pipeline of commercially viable treatments, which limits revenue generation opportunities for the sector. The disparity between research efforts and successful regulatory approvals remains significant, creating a supply bottleneck. Although the Alzheimer's Association reported 164 agents in clinical trials for Alzheimer’s disease in 2024, the high failure rate inherent to this pipeline means that despite the volume of active research, only a small fraction of these agents are likely to overcome development hurdles and contribute to actual market expansion.
Market Trends
The adoption of blood-based biomarkers is transforming patient stratification by replacing expensive and invasive diagnostic methods such as PET scans and cerebrospinal fluid analysis. This shift toward accessible plasma diagnostic tools resolves the critical bottleneck of slow clinical trial recruitment and enables earlier disease detection on a population scale, which is vital for the timely administration of therapeutics. Enhanced diagnostic precision allows for the rapid identification of suitable candidates for disease-modifying therapies, directly influencing market uptake and payer reimbursement models. According to a July 2024 press release by the Alzheimer's Association, a pivotal study demonstrated that a specific blood test known as APS2 was approximately 90% accurate in identifying Alzheimer’s pathology, significantly outperforming primary care physicians who were only 63% accurate using traditional evaluation methods.Concurrently, the sector is witnessing a strategic pivot toward repurposing GLP-1 agonists and metabolic agents, driven by the increasing recognition of the metabolic components of neurodegeneration. Pharmaceutical developers are actively investigating these agents, originally approved for diabetes and obesity, for their potential to reduce neuroinflammation and offer neuroprotection beyond traditional amyloid-targeting mechanisms. This trend represents a cost-efficient pathway to diversify therapeutic portfolios and mitigate the risks associated with novel drug development by leveraging agents with established safety profiles. According to a December 2024 BioSpace article, a real-world study indicated that patients with type 2 diabetes prescribed semaglutide showed a 40% to 70% reduction in the risk of being diagnosed with Alzheimer’s disease compared to unexposed patient groups.
Key Players Profiled in the Alzheimer’s Therapeutics Market
- Eisai Co., Ltd.
- Novartis AG
- AbbVie Inc. (Allergan plc)
- Adamas Pharmaceuticals, Inc.
- H. Lundbeck A/S
- Biogen Inc.
- AC Immune SA
- F. Hoffmann La Roche Ltd.
- Daiichi Sankyo Company Limited
- Johnson & Johnson
Report Scope
In this report, the Global Alzheimer's Therapeutics Market has been segmented into the following categories:Alzheimer's Therapeutics Market, by Product Type:
- Cholinesterase Inhibitors
- NMDA Receptor Antagonist
- Combination Drugs
- Pipeline Drugs
Alzheimer's Therapeutics Market, by End User:
- Hospital Pharmacy
- Retail Pharmacy
- E-commerce
Alzheimer's Therapeutics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Alzheimer's Therapeutics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Alzheimer's Therapeutics market report include:- Eisai Co., Ltd.
- Novartis AG
- AbbVie Inc. (Allergan PLC.)
- Adamas Pharmaceuticals, Inc.
- H. Lundbeck A/S
- Biogen Inc.
- AC Immune SA
- F. Hoffmann La Roche Ltd.
- Daiichi Sankyo Company Limited
- Johnson & Johnson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
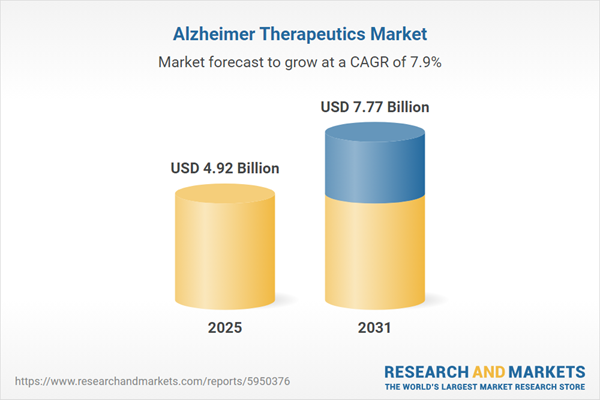
| Estimated Market Value ( USD | $ 4.92 Billion |
| Forecasted Market Value ( USD | $ 7.77 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |