Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Primary growth drivers include the increasing global incidence of seasonal influenza and the urgent need for precise, early detection to enable timely therapeutic intervention and outbreak control. Additionally, market expansion is bolstered by a growing geriatric demographic requiring regular health monitoring and a shifting preference toward rapid diagnostic solutions within decentralized healthcare environments. As reported by the Centers for Disease Control and Prevention, clinical laboratories processed roughly 3.9 million respiratory specimens for influenza during the 2024 to 2025 season, highlighting the significant volume of testing that sustains demand.
Despite these strong growth factors, the market encounters substantial hurdles due to the high costs linked to advanced molecular diagnostic platforms, which limit their uptake in resource-constrained areas. This economic barrier is often exacerbated by uneven reimbursement frameworks and the variable sensitivity associated with lower-cost rapid tests. Such limitations can result in clinical hesitation and hinder wider market penetration in cost-sensitive economies.
Market Drivers
The escalating global prevalence of both seasonal and pandemic influenza strains serves as a major market catalyst, requiring high diagnostic volumes to optimize patient care and curb transmission. The persistent mutation of viruses and the resurgence of respiratory pathogens post-pandemic have increased the disease burden, necessitating heightened vigilance from healthcare systems. For example, the 2023-2024 season saw substantial morbidity; the Centers for Disease Control and Prevention reported in November 2024 via the '2023-2024 Influenza Season Summary' that the United States experienced an estimated 40 million flu illnesses, 470,000 hospitalizations, and 28,000 deaths. This rising incidence is a global issue fueling market momentum, as confirmed by the European Centre for Disease Prevention and Control's 'Seasonal influenza - Annual Epidemiological Report for 2023/2024' from November 2024, which noted that positive influenza tests in sentinel primary care peaked at 39% in late 2023, driving global consumption of diagnostic supplies.Concurrently, there is a surging demand for rapid Point-of-Care (POC) testing solutions, spurred by the vital need for immediate clinical decisions in decentralized locations. Healthcare providers are increasingly adopting rapid molecular assays and syndromic panels that yield accurate results in minutes, thereby facilitating timely antiviral treatment and improved patient throughput. This transition toward decentralized diagnostics is highlighted by significant commercial gains; bioMérieux's 'First-Half 2024 Results' from September 2024 indicated a 17% rise in BIOFIRE respiratory panel sales during the second quarter, driven by the appeal of rapid solutions and a growing installed base. This trend signifies a structural market shift where speed and accessibility are becoming as critical as sensitivity in the management of respiratory outbreaks.
Market Challenges
The substantial cost associated with sophisticated molecular diagnostic platforms represents a major impediment to the growth of the Global Influenza Diagnostics Market, especially within resource-limited economies. Although molecular assays offer higher sensitivity than rapid immunoassays, the considerable capital investment and operational costs required for these advanced systems effectively preclude their use in many developing nations and decentralized settings. This financial inequality necessitates a continued dependence on less expensive alternatives that frequently demonstrate variable sensitivity, resulting in unreliable clinical outcomes and provider reluctance to embrace newer technologies. Consequently, the market experiences fragmented expansion, characterized by saturation of advanced diagnostics in affluent regions while vast potential markets remain underserved due to cost constraints.This disparity in diagnostic capacity is evident in recent industry data concerning global preparedness. In July 2024, the Foundation for Innovative New Diagnostics (FIND) released the Pathogen Diagnostic Readiness Index, which revealed a global readiness score of merely 56% for Influenza A and Influenza B, a figure that significantly lags behind the capacity built for SARS-CoV-2. This low score suggests that a substantial proportion of global healthcare infrastructure is missing the necessary diagnostic tools for effective management, a shortfall directly attributable to the economic challenges associated with implementing precise yet costly testing platforms.
Market Trends
The widespread implementation of Multiplex Syndromic Testing is transforming clinical workflows as healthcare systems increasingly value the simultaneous identification of co-circulating respiratory pathogens. This movement is fueled by the clinical requirement to differentiate between Influenza A, Influenza B, RSV, and SARS-CoV-2 within a single high-throughput process, which optimizes resource allocation and patient management during peak viral periods. The transition from single-target assays is gaining speed due to regulatory backing for comprehensive panels that provide efficiency while maintaining sensitivity. As reported by BioSpectrum India in February 2025, in the article 'Thermo Fisher announces 510(k) clearance of molecular clinical test..', Thermo Fisher Scientific secured FDA clearance for its Applied Biosystems TaqPath Select Panel, a multiplex solution engineered to detect and distinguish these four viral targets in one workflow, highlighting the industry's shift toward consolidated diagnostic methods.Furthermore, advancements in Lab-on-a-Chip and Microfluidic Technologies are strengthening market infrastructure by facilitating complex molecular processing inside compact, automated cartridges. These innovations allow for the miniaturization of intricate laboratory protocols, enabling precise sample preparation and amplification in decentralized settings with little need for operator involvement. The rapid growth of the installed base for these microfluidic systems suggests a structural dependence on cartridge-based ecosystems to manage variable testing loads. This scalability is demonstrated by recent operational data; according to Danaher Corporation's 'Fourth Quarter and Full Year 2024 Results' from January 2025, the global installed base for their Cepheid GeneXpert system, which utilizes advanced microfluidic cartridge technology, grew to over 60,000 instruments, underscoring the vital role of scalable technology in contemporary diagnostic strategies.
Key Players Profiled in the Influenza Diagnostics Market
- Abbott Laboratories Limited
- Becton, Dickinson and Company
- Coris BioConcept
- DiaSorin SpA
- F. Hoffmann-La Roche Ltd.
- Meridian Bioscience Inc.
- Quidel Corporation
- Sekisui Diagnostics
- Thermo Fisher Scientific Inc.
Report Scope
In this report, the Global Influenza Diagnostics Market has been segmented into the following categories:Influenza Diagnostics Market, by Test Type:
- Traditional Diagnostic Test
- Molecular Diagnostic Assay
Influenza Diagnostics Market, by End user:
- Hospital
- Laboratories
- Others
Influenza Diagnostics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Influenza Diagnostics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Influenza Diagnostics market report include:- Abbott Laboratories Limited
- Becton, Dickinson and Company
- Coris BioConcept
- DiaSorin SpA
- F. Hoffmann-La Roche Ltd
- Meridian Bioscience Inc
- Quidel Corporation
- Sekisui Diagnostics
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
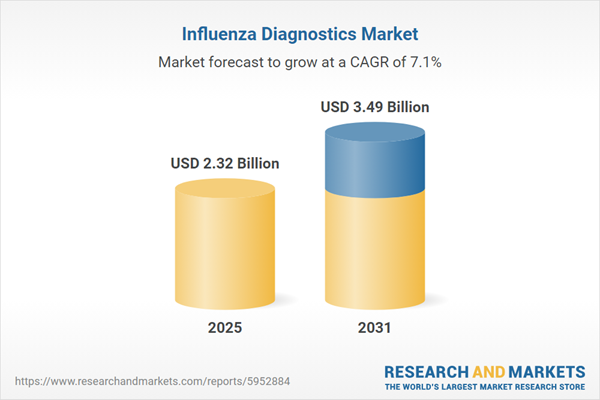
| Estimated Market Value ( USD | $ 2.32 Billion |
| Forecasted Market Value ( USD | $ 3.49 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |