Oncology is the fastest growing segment, North America is the largest market globally
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The global Real-World Evidence Solutions Market is fundamentally influenced by increasing regulatory acceptance and the profound shift towards value-based healthcare models. Regulatory bodies globally are progressively integrating real-world evidence into drug development and post-market surveillance. This evolution supports accelerated therapy development by validating outcomes derived from routine clinical practice. According to GlobeNewswire, in August 2024, 82% of drug submissions to the FDA included Real-World Evidence, up from 73% in 2022, demonstrating growing reliance on these solutions for regulatory decision-making. This emphasis by regulators highlights the need for robust real-world data collection and analysis.Key Market Challenges
The persistent issue of data interoperability and standardization across fragmented global healthcare systems directly impedes the growth of the Global Real-World Evidence Solutions Market. This challenge stems from the diverse formats, terminologies, and governance structures prevalent in real-world data generated across various clinical settings, electronic health record systems, and administrative databases worldwide. Consequently, the seamless aggregation and comprehensive analysis of this disparate data are significantly hindered, demanding extensive manual efforts and complex data harmonization processes. This operational complexity escalates the time and resources required to synthesize meaningful clinical insights, thereby diminishing the efficiency and scalability of real-world evidence initiatives.Key Market Trends
The integration of artificial intelligence (AI) and machine learning (ML) in Real-World Evidence (RWE) analytics represents a transformative trend, enhancing the ability to derive deep insights from complex datasets. These advanced technologies facilitate more efficient data processing, pattern identification, and predictive modeling, which are crucial for accelerating drug development and optimizing treatment pathways. According to eClinical Solutions' 2024 Industry Outlook, 53% of respondents from clinical operations, data management, and biometrics functions believed AI and ML would have the greatest impact on efficiency and outcomes in 2024, signifying growing industry confidence in these tools. For example, IQVIA announced a collaboration with NVIDIA in January 2025, deploying over 50 AI agents trained on 1.2 billion health records to streamline workflows in life sciences, which demonstrates how AI is directly contributing to faster drug discovery timelines and improved decision-making within the RWE solutions market.Key Market Players Profiled:
- Clinigen Group PLC
- Icon PLC
- IBM Corporation
- IQVIA Inc.
- Oracle Corporation
- Parexel International
- PerkinElmer Inc.
- Pharmaceutical Product Development (PPD Inc.)
- SAS Institute Inc.
- Syneos Health Inc.
Report Scope:
In this report, the Global Real-World Evidence Solutions Market has been segmented into the following categories:Real-World Evidence Solutions Market, By Component:
- Claims Data
- Clinical Settings Data
- Patient-Powered Data
- Pharmacy Data
- Other Components
Real-World Evidence Solutions Market, By Therapeutic Area:
- Oncology
- Immunology
- Neurology
- Cardiovascular Disease
- Other Therapeutic Areas
Real-World Evidence Solutions Market, By Region:
- North America
- Europe
- Asia Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Real-World Evidence Solutions Market .Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Clinigen Group PLC
- Icon PLC
- IBM Corporation
- IQVIA Inc.
- Oracle Corporation
- Parexel International
- PerkinElmer Inc.
- Pharmaceutical Product Development (PPD Inc.)
- SAS Institute Inc.
- Syneos Health Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2024 - 2030 |
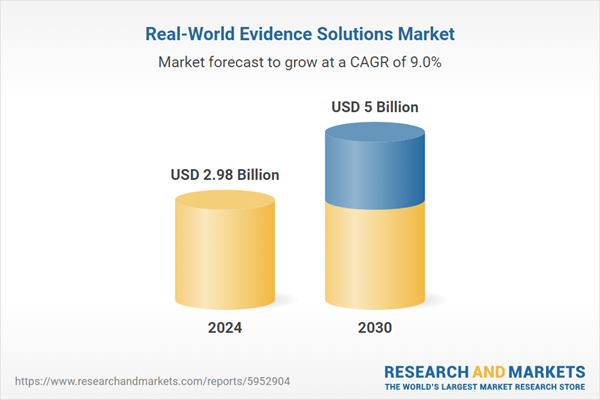
| Estimated Market Value ( USD | $ 2.98 Billion |
| Forecasted Market Value ( USD | $ 5 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |