Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite this positive growth trajectory, the market faces a significant hurdle regarding the cellular toxicity associated with many transfection methods. Chemical reagents and physical techniques frequently induce cellular stress or death, which compromises experimental validity and reduces production yields in manufacturing. Researchers often struggle to balance high delivery efficiency with cell viability, particularly when scaling operations for industrial applications. Consequently, the technical difficulty of preserving cellular health during the transfection process remains a critical obstacle that complicates the broader commercial expansion of these technologies.
Market Drivers
The rapid expansion of cell and gene therapy research acts as the primary catalyst for the global transfection reagent and equipment market. As biopharmaceutical developers transition from early-stage discovery to commercial-scale manufacturing, the requirement for high-efficiency transfection technologies to produce viral vectors and engineer cell-based therapies has intensified.This trend is directly validated by recent regulatory successes; the International Society for Cell & Gene Therapy reported in January 2025 that seven distinct cell and gene therapy products received FDA approval in 2024. Such approvals necessitate robust supply chains for transfection-grade plasmids and lipid nanoparticles, driving recurring revenue for reagent suppliers. To support this escalating commercial demand, major pharmaceutical entities are heavily expanding their production capabilities. For instance, according to Clinical Trials Arena, GSK invested $800 million in October 2024 for new drug substance and product manufacturing facilities in the United States, underscoring the massive capital flow into infrastructure that relies on advanced transfection workflows.
Growth in government initiatives and funding for biomedical sciences further accelerates the adoption of transfection technologies by lowering financial barriers for academic and industrial laboratories. Public sector grants and manufacturing funds are critical for modernizing research infrastructure, allowing scientists to procure advanced electroporation systems and novel chemical reagents.
A prime example of this fiscal support occurred when the UK Government announced a £520 million commitment in November 2024 for the Life Sciences Innovative Manufacturing Fund to bolster domestic production resilience. This influx of capital not only subsidizes the high costs associated with next-generation transfection equipment but also de-risks the development of novel non-viral delivery vectors. Consequently, state-sponsored financial stimuli are effectively widening the customer base, enabling smaller biotech firms and university hubs to integrate sophisticated transfection methods into their standard operational workflows.
Market Challenges
The primary challenge hampering the Global Transfection Reagent and Equipment Market is the inherent cellular toxicity associated with transfection methods, which directly undermines manufacturing efficiency and experimental success. When chemical reagents or physical instruments breach cell membranes to introduce nucleic acids, they frequently trigger cytotoxicity, leading to significant cell death or altered cellular behavior. This reduction in cell viability is particularly detrimental during the scale-up phase of biopharmaceutical production, where maintaining high yields of healthy cells is essential for the commercial viability of gene therapies and viral vectors. Consequently, the financial risks associated with batch failures force manufacturers to limit production runs, slowing the broader adoption of these technologies.According to the American Society of Clinical Oncology (ASCO), manufacturing data from 2024 indicated that out-of-specification (OOS) rates for certain commercial cell therapy products reached as high as 28%, with low cell viability identified as a primary contributing factor. Such high failure rates underscore the critical bottleneck caused by cytotoxic stress during production. This unreliability necessitates repeated manufacturing cycles and extensive quality control measures, inflating the cost of goods sold and creating a substantial obstacle to market expansion.
Market Trends
The transition to GMP-grade reagents for clinical manufacturing is reshaping the market as gene therapy developers increasingly demand raw materials that meet stringent regulatory standards. As therapeutic candidates progress from early research to commercialization, suppliers are aggressively acquiring specialized capabilities to provide transfection formulations compliant with Good Manufacturing Practices. This industry consolidation is evident in high-value strategic acquisitions targeting premium reagent producers to secure supply chain reliability. According to Merck KGaA, the company entered into a definitive agreement in May 2024 to acquire Mirus Bio for approximately $600 million to bolster its integrated viral vector manufacturing offer. This substantial investment underscores the critical pivot toward securing high-performance and regulatory-compliant transfection platforms essential for the scalable production of modern genetic medicines.A simultaneous shift toward non-viral delivery systems is accelerating due to the need to overcome the safety and manufacturing limitations inherent in viral vectors. Lipid nanoparticles and other chemical transfection methods are increasingly preferred for delivering mRNA and gene-editing machinery directly into patients. This technical evolution is attracting significant venture capital into companies developing next-generation in vivo delivery technologies that bypass complex ex vivo manipulation. According to BioSpace, Capstan Therapeutics raised $175 million in an oversubscribed Series B financing in March 2024 to advance its pipeline of in vivo CAR-T therapies which rely on targeted non-viral transfection. Such capital infusions highlight the growing market confidence in non-viral mechanisms as the future standard for administering complex biologic drugs.
Key Players Profiled in the Transfection Reagent and Equipment Market
- Thermo Fisher Scientific, Inc.
- Promega Corporation
- F. Hoffmann-La Roche Ltd.
- Bio-Rad Laboratories Inc.
- Merck KGaA
- OriGene Technologies Inc.
- MaxCyte, Inc.
- POLYPLUS TRANSFECTION S.A.
- Horizon Discovery Ltd.
- PromoCell GmbH
Report Scope
In this report, the Global Transfection Reagent and Equipment Market has been segmented into the following categories:Transfection Reagent and Equipment Market, by Method:
- Electroporation
- Liposomes
- Particle Bombardment
- Microinjection
- Adenoviral Vectors
- Calcium Phosphate
- DEAE-dextran
- Magnetic Beads
- Activated Dendrimers
- Laserfection
Transfection Reagent and Equipment Market, by Application:
- Gene Expression Studies
- Protein Production
- Transgenic Models
- Therapeutic Delivery
- Cancer Research
- Biomedical Research
Transfection Reagent and Equipment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Transfection Reagent and Equipment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Transfection Reagent and Equipment market report include:- Thermo Fisher Scientific, Inc.
- Promega Corporation
- F. Hoffmann-La Roche Ltd.
- Bio-Rad Laboratories Inc.
- Merck KGaA
- OriGene Technologies Inc.
- MaxCyte, Inc.
- POLYPLUS TRANSFECTION S.A.
- Horizon Discovery Ltd.
- PromoCell GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
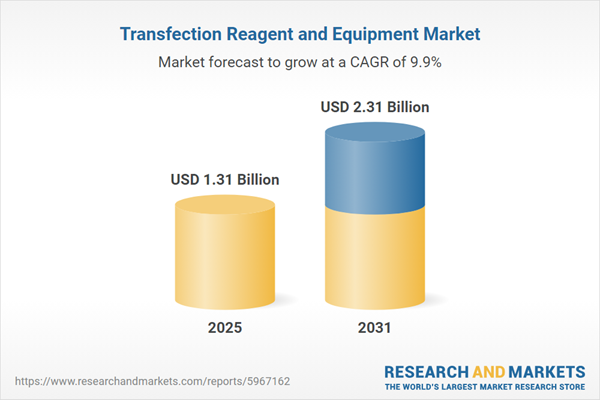
| Estimated Market Value ( USD | $ 1.31 Billion |
| Forecasted Market Value ( USD | $ 2.31 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |