Speak directly to the analyst to clarify any post sales queries you may have.
Concise foundational orientation to unsaturated iron-binding capacity testing that frames clinical relevance, assay context, and operational implications for diagnostic stakeholders
The unsaturated iron-binding capacity (UIBC) parameter has emerged as a critical adjunct to iron metabolism assessment, providing clinicians and laboratorians with refined insight into transferrin binding potential and subtle dysregulation states. UIBC testing supports differential diagnosis in complex anemias, iron overload conditions, and inflammatory states where total iron and ferritin values alone may be ambiguous. As laboratory workflows evolve toward higher throughput and integrated diagnostic panels, UIBC assays have gained renewed attention for their ability to complement complementary biomarkers and clarify clinical context.Across clinical pathways, the value proposition of UIBC lies in its relative resilience to acute phase shifts and its compatibility with automated diagnostic platforms. Consequently, laboratories are evaluating UIBC within broader biomarker portfolios and considering implications for instrumentation, reagent selection, and quality assurance. This introduction frames subsequent analysis by situating UIBC within contemporary diagnostic priorities, highlighting how assay characteristics interplay with patient care objectives and laboratory operational constraints.
Overview of pivotal technological, clinical, and service delivery shifts reshaping unsaturated iron-binding capacity testing practices and laboratory priorities
The landscape for unsaturated iron-binding capacity testing is undergoing transformative shifts driven by technology maturation, changing clinical practice patterns, and evolving laboratory business models. Advances in analyzer automation and integration have reduced hands-on time while increasing reproducibility, enabling broader adoption across high-volume hospital laboratories and centralized reference facilities. Concurrently, reagent developers are optimizing kit chemistries for robustness across sample types and interfering substances, improving concordance with complementary iron panel assays.Beyond instrumentation and reagent innovation, service delivery is shifting as contract research organizations and reference laboratories expand value-added reporting and consultative services that help clinicians interpret complex iron indices. The diffusion of digital laboratory platforms and connectivity standards further accelerates adoption by enabling seamless data exchange and downstream decision support. Together, these shifts are reorganizing how UIBC testing is procured, performed, and interpreted, creating new opportunities for standardization, clinical utility studies, and cross-disciplinary collaboration between laboratory medicine and clinical specialties.
Analysis of cumulative import tariff impacts on procurement strategies, supply chains, and cross-vendor validation requirements for UIBC testing within the United States
Recent tariff measures affecting imports into the United States have introduced nuanced operational considerations for laboratories that rely on internationally sourced analyzers, reagent kits, and specialized consumables. Procurement teams have responded by reassessing supplier diversification, inventory policies, and total landed cost calculations to preserve testing continuity. Where tariffs have increased the relative cost of imported instruments or reagents, some organizations have accelerated evaluation of domestic suppliers, public-private partnerships, or multi-vendor strategies to mitigate single-source exposure.Importantly, tariff-driven procurement decisions have downstream implications for assay validation timelines and technical support relationships. Laboratories transitioning between suppliers to manage tariff impacts must ensure rigorous comparative verification and harmonization of reference ranges to avoid diagnostic drift. In parallel, manufacturers and distributors are revising distribution agreements and logistical approaches to maintain service levels, preserve reagent shelf life during extended supply chains, and support regulatory documentation required for clinical use. These cumulative adaptations underscore the need for resilient sourcing strategies and robust cross-vendor validation protocols in an environment of trade policy volatility.
In-depth segmentation synthesis linking product architecture, end-user priorities, analytical technologies, methods, and sample protocols to strategic adoption pathways and operational fit
Segmentation analysis reveals how product architecture, end-user context, technological approach, methodological design, and sample handling protocols interact to shape adoption pathways and service models. Product type distinctions between analyzers, reagent kits, and services clarify where capital expenditure, consumable procurement, and outsourced testing responsibilities fall; within analyzers, the split between automated and semi-automated platforms guides throughput and staffing models, while reagent kits differentiating multiplex kits from single-analyte kits, and further distinguishing 2-plex from 3+-plex options, affect panel design and clinical workflow alignment. Services segmentation that separates contract research from reference laboratory offerings highlights opportunities for specialized reporting and scalable testing capacity.End users such as academic centers, diagnostic laboratories, and hospitals have distinct priorities that influence procurement and implementation. Academic centers with research institutes and universities prioritize methodological flexibility and assay validation potential; diagnostic labs, whether hospital-owned or independent, balance throughput, turnaround time, and reimbursement dynamics; hospitals categorized as secondary or tertiary centers focus on integration with inpatient care pathways and specialty consultative needs. Technology segmentation into chemiluminescence, colorimetric, and turbidimetric approaches, with subcategories like CLIA versus ECL, continuous flow versus discrete analyzers, and end point versus kinetic turbidimetry, determines analytical sensitivity, instrument footprint, and compatibility with existing platforms. Method distinctions between automated and manual workflows, including fully automated, semi-automated, and manual spectrophotometric approaches, drive labor allocation and quality control practices. Finally, sample-type considerations between plasma and serum, and within plasma the differentiation of EDTA and heparin matrices, influence preanalytical SOPs and assay calibration decisions. Taken together, this layered segmentation provides a structured lens for evaluating technology fit, operational readiness, and incremental investment pathways for institutions considering or expanding UIBC testing capabilities.
Comprehensive regional assessment of laboratory infrastructure, regulatory diversity, and procurement behavior shaping unsaturated iron-binding capacity testing across global regions
Regional dynamics exert a strong influence on regulatory processes, reimbursement frameworks, and laboratory network configurations that determine how UIBC testing is deployed. In the Americas, a combination of integrated health systems and centralized reference laboratory networks fosters demand for high-throughput automated analyzers and standardized reagent kits, while procurement sensitivity to trade policy and local manufacturing capacity affects sourcing choices and supply chain risk management. Transitioning north to south, laboratory consolidation trends and public health screening priorities shape the balance between in-house testing at tertiary centers and outsourced services to specialized reference labs.Across Europe, the Middle East & Africa, heterogeneous regulatory regimes and variable laboratory infrastructures create differentiated uptake patterns that favor flexible assay formats and adaptable instrumentation. In some regions, clinical guideline alignment and investments in hospital laboratory modernization accelerate adoption of chemiluminescence and discrete analyzer technologies, whereas in others resource constraints tip preferences toward colorimetric or manual spectrophotometric solutions that are cost-efficient and easier to maintain. In the Asia-Pacific region, rapid expansion of diagnostic capacity, rising clinician demand for comprehensive iron panels, and active local manufacturing of reagents and analyzers converge to support a diverse ecosystem; high-volume centers are investing in fully automated systems to meet throughput needs while smaller facilities prioritize semi-automated or reagent-optimized approaches to balance affordability and performance.
Strategic competitive overview highlighting how instrumentation leaders, reagent innovators, and service providers are shaping assay performance, interoperability, and value-added support
The competitive landscape features a combination of global diagnostics firms, specialized reagent developers, and service-oriented reference laboratories that collectively drive product-level innovation and application-specific services. Large analyzer manufacturers continue to invest in automation, connectivity, and multi-assay compatibility to offer end users integrated solutions that reduce footprint and streamline workflow. Reagent innovators are focusing on assay robustness, multiplexing capability, and simplified calibration procedures to support cross-platform interoperability and reduce analytical variability across heterogeneous laboratory environments.Service providers, including contract research organizations and centralized reference labs, are expanding consultative offerings and interpretive reporting to translate complex iron indices into actionable clinical narratives. Collaboration between instrument vendors and service specialists is increasingly common, enabling bundled propositions that combine hardware, consumables, and outsourced testing contracts. These strategic interactions are fostering competitive differentiation based on service reliability, local technical support, and evidence supporting clinical utility rather than purely on price. As a result, institutions evaluating UIBC adoption must weigh vendor ecosystems, lifecycle support commitments, and the availability of collaborative validation programs when selecting partners.
Practical, high-impact recommendations for manufacturers, laboratory leaders, and service providers to strengthen resilience, clinical evidence, and adoption pathways for UIBC testing
Industry leaders should pursue a set of coordinated actions that strengthen resilience, enhance clinical utility, and accelerate adoption of UIBC testing across care pathways. First, invest in interoperability and cross-platform harmonization efforts that reduce friction for laboratories transitioning between reagent suppliers or analyzer models; establishing standardized reference procedures and collaborative validation protocols will lower barriers to adoption and mitigate diagnostic variability. Second, augment clinical evidence through multicenter studies and real-world data programs that demonstrate how UIBC improves diagnostic clarity in targeted indications, thereby facilitating inclusion in clinical guidelines and strengthening payer conversations.Additionally, prioritize service models that combine product supply with interpretive reporting and training to support uptake in diverse end-user contexts. Build flexible commercial arrangements that accommodate varied procurement preferences-ranging from reagent-only agreements to bundled hardware-service contracts-and incorporate contingency planning for supply chain disruptions. Finally, engage proactively with regulatory bodies and clinical networks to align assay performance claims with evolving diagnostic standards, ensuring that product development roadmaps reflect both scientific advances and practical laboratory constraints. These recommendations, executed in concert, will help organizations translate technological capability into sustainable clinical impact.
Methodological framework describing primary stakeholder engagement, technical synthesis, and validation steps used to derive actionable insights on UIBC testing operations
The research underpinning this analysis integrates primary stakeholder engagement, technical literature synthesis, and systematic review of regulatory and operational trends. Primary inputs included structured interviews with laboratory directors, clinical pathologists, procurement leads, and technical service managers to capture real-world workflow constraints, validation experiences, and procurement considerations. These qualitative insights were triangulated with technical assay performance data from peer-reviewed journals, manufacturer technical documentation, and white papers that describe analytic principles and preanalytical variables relevant to UIBC measurement.To ensure analytical rigor, comparative assessments of technologies considered sensitivity, specificity, throughput, and compatibility with discrete clinical workflows, while validation considerations examined cross-platform harmonization challenges and sample matrix effects such as differences between EDTA and heparin plasma or serum. Supply chain and procurement analyses incorporated publicly available trade policy updates and industry reports on manufacturing footprints to evaluate sourcing risk. Throughout the methodology, iterative validation cycles were used to reconcile divergent stakeholder perspectives and surface consensus on operational priorities, ensuring the conclusions and recommendations are grounded in practice-based evidence.
Concluding synthesis that ties diagnostic capability, operational readiness, and strategic alignment to elevate the role of UIBC testing in clinical decision-making
In summary, unsaturated iron-binding capacity testing occupies a pivotal role in nuanced iron diagnostics by offering complementary information that enhances clinical interpretation in complex cases. Technological advances in automation, reagent design, and analytical chemistry have improved assay robustness, enabling broader deployment across varied laboratory settings. At the same time, evolving procurement dynamics and regional healthcare structures necessitate adaptable implementation strategies that reconcile throughput requirements, budgetary constraints, and regulatory expectations.Looking forward, the most successful adopters will be those who combine rigorous validation and harmonization practices with collaborative vendor relationships and data-driven clinical evidence generation. By aligning technical capabilities with service-oriented delivery models and resilient sourcing strategies, laboratories and their partners can embed UIBC testing more effectively into diagnostic pathways, improving patient care while maintaining operational efficiency.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Unsaturated Iron-Binding Capacity Analysis Market
Companies Mentioned
The key companies profiled in this Unsaturated Iron-Binding Capacity Analysis market report include:- Abbott Laboratories Inc.
- Arkray Inc.
- Beckman Coulter Inc.
- Bio-Rad Laboratories Inc.
- Boditech Med Inc.
- DiaSorin S.p.A.
- Diatron MI Zrt.
- Erba Mannheim
- Fujirebio Inc.
- HORIBA ABX SAS
- Horiba Ltd.
- Mindray Medical International Limited
- Nova Biomedical Corporation
- Randox Laboratories Ltd.
- Roche Diagnostics International Ltd.
- Siemens Healthineers AG
- Snibe Diagnostic Co. Ltd.
- Sysmex Corporation
- Tosoh Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
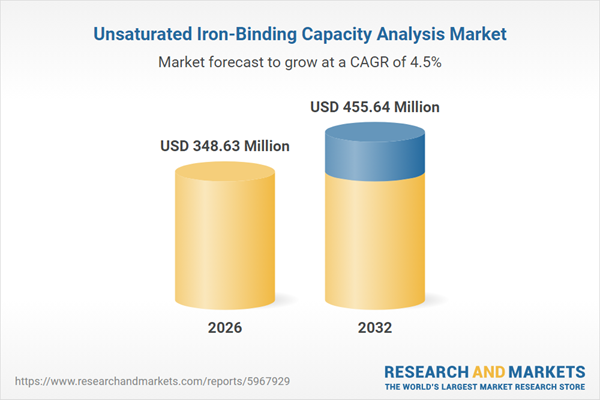
| Estimated Market Value ( USD | $ 348.63 Million |
| Forecasted Market Value ( USD | $ 455.64 Million |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |