The myasthenia gravis market has been comprehensively analyzed in this report titled "Myasthenia Gravis Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Myasthenia gravis (MG) is a chronic autoimmune condition which weakens and exhausts muscles, particularly those that control facial expression, eye movement, and swallowing. The loss of acetylcholine receptors at the neuromuscular junction reduces the regular transmission of nerve impulses to the muscles, hence leading to weakness. The signs and symptoms of MG can vary from person to person and can appear suddenly or gradually. Some of the common symptoms of MG include fatigue, muscle weakness, droopy eyelids, shortness of breath, difficulty in speaking, rapid eye movements, muscle twitching, etc. The diagnosis of MG generally involves a combination of clinical evaluations, tests, and procedures, such as blood tests, electromyography (EMG), MRI, and CT scan. Additionally, myasthenia gravis can also be identified using a diagnostic test known as the Edrophonium (Tensilon) test, which involves temporarily administering a drug to increase muscle strength. Some additional tests might also be required to assess the type and severity of MG and track the disease's development.
The rising prevalence of autoimmune illnesses and viral infections, such as the Epstein-Barr virus and cytomegalovirus, coupled with the growing unmet need for treatments, is primarily driving the global myasthenia gravis market. In addition to this, the escalating utilization of cholinesterase inhibitors, which assist in improving muscle strength by increasing the availability of acetylcholine at the neuromuscular junction, to treat the ailment is creating a positive outlook for the market. Furthermore, the widespread adoption of immunosuppressive therapy to suppress the immune system and reduce the production of antibodies that attack the acetylcholine receptors, is also bolstering the market growth. Besides this, the emerging popularity of plasmapheresis therapy, which involves removing the plasma from the blood and replacing it with intravenous fluids to remove harmful antibodies for treating MG is acting as a significant growth-inducing factor. Moreover, the introduction of several initiatives by numerous government bodies across the globe to create awareness regarding myasthenia gravis and support faster approvals of pipeline drugs and clinical trials that encourage the entry of new products is further augmenting the global market. Additionally, various key players are making extensive investments in developing monoclonal antibodies that block the activity of complement proteins involved in the immune response. This, in turn, is expected to drive the global myasthenia gravis in the coming years.
This report provides an exhaustive analysis of the myasthenia gravis market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for Myasthenia gravis and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the myasthenia gravis market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the myasthenia gravis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the myasthenia gravis market
Competitive Landscape:
This report also provides a detailed analysis of the current myasthenia gravis marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the myasthenia gravis market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the myasthenia gravis market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the myasthenia gravis market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of myasthenia gravis across the seven major markets?
- What is the number of prevalent cases (2018-2034) of myasthenia gravis by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of myasthenia gravis by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of myasthenia gravis by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with myasthenia gravis across the seven major markets?
- What is the size of the myasthenia gravis patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of myasthenia gravis ?
- What will be the growth rate of patients across the seven major markets?
Myasthenia Gravis: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for myasthenia gravis drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the myasthenia gravis market?
- What are the key regulatory events related to the myasthenia gravis market?
- What is the structure of clinical trial landscape by status related to the myasthenia gravis market?
- What is the structure of clinical trial landscape by phase related to the myasthenia gravis market?
- What is the structure of clinical trial landscape by route of administration related to the myasthenia gravis market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 134 |
| Published | May 2024 |
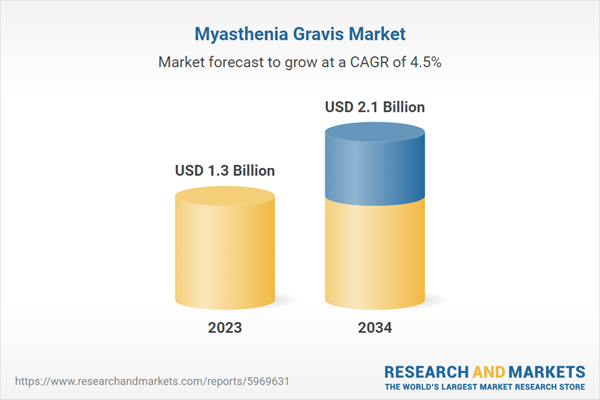
| Forecast Period | 2023 - 2034 |
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 2.1 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |