The drug-resistant epilepsy market has been comprehensively analyzed in this report titled "Drug-Resistant Epilepsy Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Drug-resistant epilepsy, also known as refractory epilepsy, refers to a form of neurological disorder in which seizures are not adequately controlled or eliminated by anti-seizure medications. The disease is characterized by recurrent seizures, that are caused by abnormal electrical activity in the brain. This condition can have a significant impact on the patient's quality of life, affecting their physical and mental well-being, ability to work or drive, and overall daily functioning. The most common indications associated with the illness include visual disturbance, unusual taste, muscle weakness, difficulty speaking, numbness, tingling, sensory or motor impairment, memory problems, mood swings, irritability, depression, low blood sugar, fainting, etc. The diagnosis of drug-resistant epilepsy is mainly based on a combination of clinical feature evaluation, medical history assessment, physical examination, and neuropsychological tests. An electroencephalogram is also used, which helps in detecting abnormal brain wave patterns and identifying the location and type of seizures. The healthcare provider may further perform several diagnostic studies, such as single-photon emission computed tomography and magnetic resonance imaging, to visualize metabolic activities in the brain.
The rising cases of brain structural abnormalities, like tumors and vascular malformations, resulting from previous brain injuries or surgeries, which can alter the electrical network and interfere with the effectiveness of anti-seizure medications, are primarily driving the drug-resistant epilepsy market. In addition to this, the increasing incidences of inherited defects, that cause genetic variations in drug-metabolizing enzymes, drug transporters, or target receptors are also propelling the market growth. Moreover, the escalating application of effective therapeutic agents, including cannabidiol, felbamate, vigabatrin, etc., to manage the symptoms of drug-resistant epilepsy is creating a positive outlook for the market. Additionally, the inflating demand for neurofeedback therapy, a non-invasive technique to potentially encourage self-regulation and increase independence in daily activities of patients, is acting as another significant growth-inducing factor. Furthermore, the widespread adoption of neuromodulation devices, such as vagus nerve stimulation, responsive neurostimulation, deep brain stimulation, etc., since they continuously monitor brain activity and deliver electrical stimulation to the affected regions in order to disrupt the abnormal brain patterns and reduce seizures, is expected to drive the drug-resistant epilepsy market in the coming years.
This report provides an exhaustive analysis of the drug-resistant epilepsy market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for drug-resistant epilepsy and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the drug-resistant epilepsy market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the drug-resistant epilepsy market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the drug-resistant epilepsy market
Competitive Landscape:
This report also provides a detailed analysis of the current drug-resistant epilepsy marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the drug-resistant epilepsy market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the drug-resistant epilepsy market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the drug-resistant epilepsy market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of drug-resistant epilepsy across the seven major markets?
- What is the number of prevalent cases (2018-2034) of drug-resistant epilepsy by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of drug-resistant epilepsy by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with drug-resistant epilepsy across the seven major markets?
- What is the size of the drug-resistant epilepsy patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of drug-resistant epilepsy?
- What will be the growth rate of patients across the seven major markets?
Drug-Resistant Epilepsy: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for drug-resistant epilepsy drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the drug-resistant epilepsy market?
- What are the key regulatory events related to the drug-resistant epilepsy market?
- What is the structure of clinical trial landscape by status related to the drug-resistant epilepsy market?
- What is the structure of clinical trial landscape by phase related to the drug-resistant epilepsy market?
- What is the structure of clinical trial landscape by route of administration related to the drug-resistant epilepsy market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 137 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
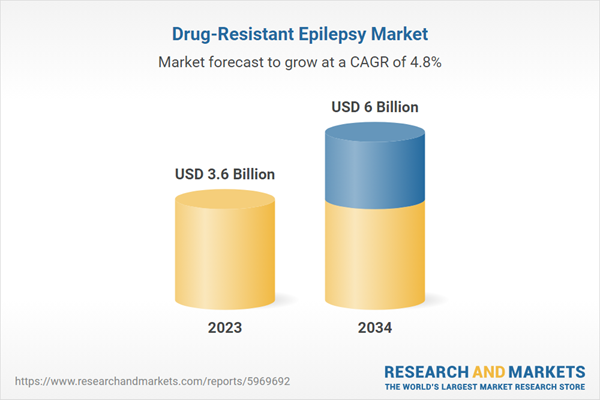
| Estimated Market Value ( USD | $ 3.6 Billion |
| Forecasted Market Value ( USD | $ 6 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |