The Fuchs dystrophy market has been comprehensively analyzed in this report titled "Fuchs Dystrophy Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Fuchs dystrophy is a progressive eye disease that damages the cornea, which is the transparent, dome-shaped surface that covers the front of the eye. It is a hereditary condition that affects women more frequently than men. The main symptom of the ailment is gradual loss of vision, which typically begins in the morning and improves throughout the day. As the condition progresses, patients may experience increased sensitivity to light, blurred vision, and difficulty seeing at night. In advanced cases, fluid may accumulate in the cornea, causing swelling and distortion of vision. Fuchs dystrophy can be diagnosed through a comprehensive eye exam performed by an ophthalmologist. During the exam, the doctor will evaluate the patient's vision and check for signs of corneal swelling or thickening. They also use a special microscope called a slit lamp to examine the cornea in detail and assess the health of the cells that line its surface. In addition to this, the doctor may perform specular microscopy, which uses a special camera to create a high-resolution image of the cornea's inner layer. This can help to detect changes in the cells that may indicate the presence of Fuchs dystrophy. In a few cases, genetic testing is also recommended to confirm the diagnosis.
The increasing cases of genetic mutations that affect the production of proteins in the cornea, which further lead to progressive thinning and clouding of the cornea, are primarily driving the Fuchs dystrophy market. In addition to this, the inflating adoption of steroids and nonsteroidal anti-inflammatory drugs to reduce inflammation and swelling in the cornea, thereby improving vision and easing discomfort, is also bolstering the market growth. Moreover, the rising application of phototherapeutic keratectomy (PTK) to remove excess corneal tissue and smooth out the surface of the cornea is acting as another significant growth-inducing factor. Apart from this, the escalating demand for bandage contact lenses, since they protect the cornea from further damage by providing a smooth surface for the eyelid to glide over, is creating a positive outlook for the market. They also aid in alleviating swelling and improving vision by drawing excess fluid from the cornea. Additionally, the emerging popularity of Descemet membrane endothelial keratoplasty on account of its several associated benefits, such as reduced risk of rejection, lowered likelihood of astigmatism, minimal impact on the cornea, faster recovery time, etc., is expected to drive the Fuchs dystrophy market in the coming years.
This report provides an exhaustive analysis of the Fuchs dystrophy market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for Fuchs dystrophy and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the Fuchs dystrophy market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Fuchs dystrophy market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Fuchs dystrophy market
Competitive Landscape:
This report also provides a detailed analysis of the current Fuchs dystrophy marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the Fuchs dystrophy market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the Fuchs dystrophy market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the Fuchs dystrophy market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of Fuchs dystrophy across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Fuchs dystrophy by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Fuchs dystrophy by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with Fuchs dystrophy across the seven major markets?
- What is the size of the Fuchs dystrophy patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of Fuchs dystrophy?
- What will be the growth rate of patients across the seven major markets?
Fuchs Dystrophy: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Fuchs dystrophy drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Fuchs dystrophy market?
- What are the key regulatory events related to the Fuchs dystrophy market?
- What is the structure of clinical trial landscape by status related to the Fuchs dystrophy market?
- What is the structure of clinical trial landscape by phase related to the Fuchs dystrophy market?
- What is the structure of clinical trial landscape by route of administration related to the Fuchs dystrophy market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
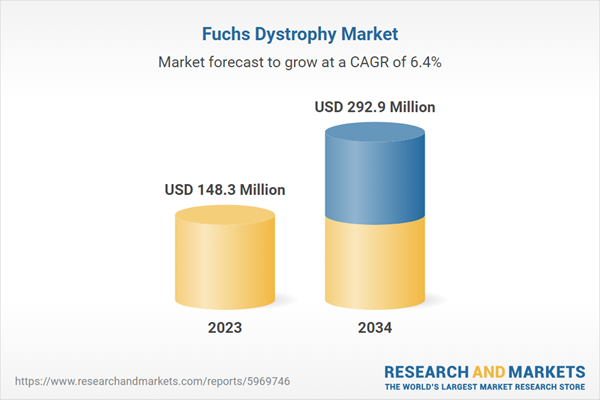
| Estimated Market Value ( USD | $ 148.3 Million |
| Forecasted Market Value ( USD | $ 292.9 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |