The hepatocellular carcinoma market has been comprehensively analyzed in this report titled "Hepatocellular Carcinoma Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Hepatocellular carcinoma (HCC) is a type of oncological disease that arises from the hepatocytes or liver cells. It is a severe illness that can destroy hepatic cells and interfere with the liver's ability to function, causing life-threatening complications. The symptoms of the ailment vary depending on the stage and size of the tumor, and many people may not experience any indications at all. Some of the non-specific symptoms include abdominal pain, nausea, vomiting, tiredness, etc. Individuals suffering from hepatocellular carcinoma may also experience yellow skin, loss of appetite, abdominal swelling, unintentional weight loss, easy bruising, etc. The diagnosis of this ailment is typically based on the patient's symptoms, blood testing, and imaging evaluation. The healthcare provider may also perform numerous diagnostic procedures, such as multi-phase computerized tomography scans and contrast-enhanced magnetic resonance imaging, to detect disease staging among patients. Additionally, a biopsy or ultrasound of the tumor is required to confirm the diagnosis of hepatocellular carcinoma.
The increasing incidences of chronic hepatitis B and C infections, which cause long-term liver inflammation and lead to the development of cirrhosis, are primarily driving the hepatocellular carcinoma market. In addition to this, the rising prevalence of several associated risk factors, including excessive alcohol consumption, obesity, diabetes, accumulation of fat in the liver, etc., is also bolstering the market growth. Furthermore, the widespread adoption of monoclonal antibodies for treating unresectable or advanced disease conditions is acting as another significant growth-inducing factor. This medication can prevent the proliferation of unhealthy cells, thereby reducing tumors' ability to grow and metastasize. Additionally, the escalating demand for locoregional therapies, such as thermal ablation and transarterial chemoembolization, that aim to decrease tumor viability, delay disease progression, and ultimately extend overall survival is also creating a positive outlook for the market. Moreover, the inflating utilization of selective internal radiation therapy, which potently delivers millions of tiny radioactive beads directly to liver tumors and provides cancer irradiation, is further augmenting the market growth. Apart from this, the emerging popularity of percutaneous ethanol injection among patients with early-stage HCC who are not candidates for surgery or other more invasive treatments is expected to drive the hepatocellular carcinoma market in the coming years.
This report provides an exhaustive analysis of the hepatocellular carcinoma market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for hepatocellular carcinoma and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the hepatocellular carcinoma market in any manner.
Recent Developments:
In April 2024, Geneos Therapeutics released positive findings from its Phase I/II GT-30 clinical trial of GNOS-PV02, a personalized neoantigen vaccine, in combination with pembrolizumab for advanced hepatocellular carcinoma. The data demonstrated that nearly a third of the patients treated with this combination therapy observed tumor shrinkage, with three of those individuals attaining a complete response, which means no visible tumor signs remained after a median follow-up of 21.5 months.
In November 2023, SCG Cell Therapy Pte Ltd (SCG) presented late-breaking clinical data from its first-in-class autologous hepatitis B virus (HBV)-specific T-cell receptor-engineered T Cell (TCR-T) treatment, SCG101, at the 2023 AASLD Liver Meeting in Boston, United States. In a first-in-human clinical trial, SCG101 displayed potential antiviral and anticancer activity in patients with advanced HBV-related hepatocellular carcinoma.
In June 2023, Tvardi Therapeutics, Inc. disclosed that the first patients had been dosed in every arm of its ongoing REVERT LIVER CANCER study. The trial evaluates the safety and therapeutic activity of TTI-101 as monotherapy and in conjunction with standard-of-care therapy in patients with locally advanced or metastatic and unresectable hepatocellular carcinoma.
In February 2023, Genoscience Pharma reported that its leading candidate, ezurpimtrostat, a PPT-1 (Palmitoyl Protein Thioesterase-1) inhibitor, has received Orphan Drug Designation (ODD) from the US FDA for the treatment of hepatocellular carcinoma.
Key Highlights:
Hepatocellular carcinoma is diagnosed in around 900,000 people globally each year.
It is the fifth most prevalent malignancy in men (69.8% of all hepatocellular carcinoma cases) and the ninth among women.
According to the Global Cancer Observatory (GCO), Asia accounts for 72.5% of all HCC cases, with 11.6 new cases per 100,000 population.
HCC is less prevalent in the US and Europe, with 5.2 new cases per 100,000 people per year.
About 830,000 individuals die from HCC annually, making it the third most common cause of cancer-related deaths worldwide.
The Surveillance, Epidemiology, and End Results (SEER) Program indicated a 20.3% 5-year relative survival rate for individuals diagnosed with HCC.
Drugs:
KEYTRUDA is an anti-programmed death receptor-1 (PD-1) medication that enhances the immune system's ability to detect and attack tumor cells. KEYTRUDA is a humanized monoclonal antibody that inhibits the interaction of PD-1 and its ligands, PD-L1 and PD-L2, activating T lymphocytes that can affect both tumor and healthy cells.
GC33 is a humanized monoclonal antibody created by Chugai. The drug targets glypican-3 (GPC3), which is specifically expressed in hepatocellular carcinoma.
TTI-101 is under clinical development by Tvardi Therapeutics for metastatic hepatocellular carcinoma. It is an oral small molecule STAT3 inhibitor. STAT3 is a regulatory molecule that contributes considerably to hepatocellular carcinoma progression.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hepatocellular carcinoma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hepatocellular carcinoma market
Competitive Landscape:
This report also provides a detailed analysis of the current hepatocellular carcinoma marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the hepatocellular carcinoma market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the hepatocellular carcinoma market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the hepatocellular carcinoma market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of hepatocellular carcinoma across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hepatocellular carcinoma by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hepatocellular carcinoma by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with hepatocellular carcinoma across the seven major markets?
- What is the size of the hepatocellular carcinoma patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of hepatocellular carcinoma?
- What will be the growth rate of patients across the seven major markets?
Hepatocellular Carcinoma: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for hepatocellular carcinoma drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the hepatocellular carcinoma market?
- What are the key regulatory events related to the hepatocellular carcinoma market?
- What is the structure of clinical trial landscape by status related to the hepatocellular carcinoma market?
- What is the structure of clinical trial landscape by phase related to the hepatocellular carcinoma market?
- What is the structure of clinical trial landscape by route of administration related to the hepatocellular carcinoma market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 138 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
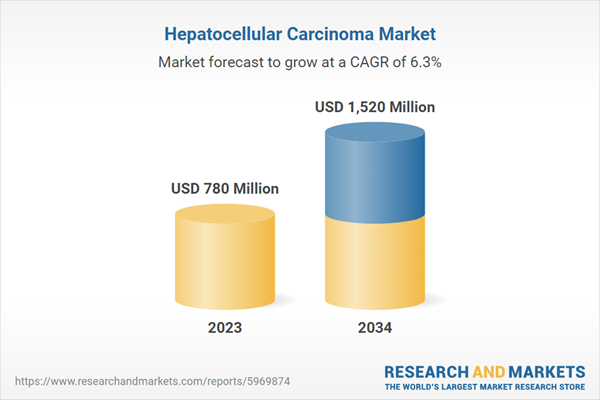
| Estimated Market Value ( USD | $ 780 Million |
| Forecasted Market Value ( USD | $ 1520 Million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |