The Friedreich's ataxia market has been comprehensively analyzed in this report titled "Friedreich's Ataxia Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Genital warts refer to a sexually transmitted infection caused by a low-risk strain of human papillomavirus. The condition results in the formation of tiny bumps or growths in and around the genitals and rectum. Warts typically appear following one to eight months after exposure. The disease is transmitted through skin-to-skin contact and sexual activities, including vaginal, oral, and anal sex. The common indications associated with Friedreich's ataxia are mild bleeding, discomfort, burning sensation, itching, irritation, etc. This ailment often does not produce significant physical markers, but an outbreak might result in emotional problems like anxiety in some people. The diagnosis typically involves a review of the patient's medical history, underlying symptoms, and physical examination. The healthcare practitioner may also perform an acetowhite test, which helps to make Friedreich's ataxia more visible. Additionally, various diagnostic procedures, such as colposcopy and biopsy, are required to visually examine the cervix, vagina, and vulva in order to confirm a diagnosis.
The increasing prevalence of human papillomavirus infections caused by the non-usage of condoms or dental dams during sex is primarily driving the Friedreich's ataxia market. In addition to this, the rising incidence of several associated risk factors, such as a weakened immune system, multiple sexual partners, engaging in sexual activity at an early age, etc., is also bolstering the market growth. Furthermore, the widespread utilization of topical immunomodulatory agents, including imiquimod and cidofovir, to stimulate the immune system in order to attack the virus that causes the warts is acting as another significant growth-inducing factor. Apart from this, the inflating application of cryosurgery, which utilizes liquid nitrogen to freeze and disrupt warts, is further creating a positive outlook for the market. Moreover, the emerging popularity of loop electrosurgical excision procedures for treating the condition is also propelling the market growth. This method uses a thin, low-voltage, electrically charged wire loop to separate warts from the skin by heating the margin of the affected area. Additionally, the escalating demand for interferon alfa-n3 injection to manage the ailment, since it can be administered directly into the lesion with a quicker onset of action, is expected to drive the Friedreich's ataxia market in the coming years.
This report provides an exhaustive analysis of the Friedreich's ataxia market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for Friedreich's ataxia and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the Friedreich's ataxia market in any manner.
Recent Developments:
In March 2024, Larimar Therapeutics, Inc. announced the dosing of the first patient in an open-label extension (OLE) trial assessing nomlabofusp subcutaneous injections at a dose of 25 mg per day for individuals with Friedreich's ataxia. Nomlabofusp (CTI-1601) is a new protein replacement therapy that targets the underlying cause of Friedreich's ataxia by delivering frataxin to the mitochondria.
In March 2024, Lexeo published preliminary findings from a subset of their second dose cohort of the SUNRISE-FA trial, demonstrating a rise in frataxin levels three months after treatment with LX2006 compared to pre-treatment baseline levels for people with Friedreich's ataxia cardiomyopathy.
In February 2024, Voyager Therapeutics, Inc. announced that the joint steering committee with Neurocrine Biosciences had chosen a lead development candidate for Friedreich's ataxia program. The candidate combines a frataxin (FXN) gene replacement payload with an intravenous, blood-brain barrier penetrant, new capsid from Voyager's TRACER capsid discovery platform.
In February 2024, Biogen Inc. announced that the European Commission (EC) had approved SKYCLARYS (omaveloxolone) for the treatment of Friedreich's ataxia in adults and adolescents aged 16 and above. SKYCLARYS is the first medication approved in the European Union for this rare, genetically determined, progressive neurodegenerative disease.
Key Highlights:
Friedreich's ataxia affects one in every 50,000 people in the United States, with Western Europeans having the highest prevalence.
The worldwide prevalence of Friedreich's ataxia is 1 in 40,000.
Friedreich's ataxia is a disease that affects young people, as the development of symptoms usually begins before the age of 20.
Friedreich's ataxia is the most prevalent inherited ataxia in the Caucasian population. The estimated incidence in Caucasians is around 1 in 29,000, with a carrier chance of 1 in 85.
Approximately 7% of patients with Friedreich's ataxia have diabetes.
Drugs:
Skyclarys (omaveloxolone) is FDA-approved medication in the United States for the treatment of Friedreich's ataxia in adults and adolescents aged 16 and above. The recommended dose is 150 mg to be administered orally once daily. Skyclarys is intended to stimulate nuclear factor erythroid 2-related factor 2 (NrF2), a transcription factor whose signaling is reduced in Friedreich's ataxia patients. NrF2 activates genes that improve mitochondrial function, increase antioxidant responses, and reduce inflammation.
Vatiquinone is a small molecule, first-in-class selective inhibitor of 15-Lipoxygenase (15-LO), an enzyme that regulates the energy and oxidative stress pathways that are disturbed in Friedreich's ataxia. Inhibiting 15-LO helps to reduce the effects of mitochondrial malfunction and oxidative stress, hence reducing ferroptosis and promoting neuronal survival.
Leriglitazone (MIN-102) is an orally bioavailable and selective PPARγ agonist with a potential best-in-class and first-in-class profile indicated for CNS diseases. It has demonstrated significant brain penetration and an acceptable safety profile. The medicine provided strong preclinical proof-of-concept in animal models of Friedreich's ataxia by changing pathways that lead to neuroinflammation, demyelination, and mitochondrial failure.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Friedreich's ataxia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Friedreich's ataxia market
Competitive Landscape:
This report also provides a detailed analysis of the current Friedreich's ataxia marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the Friedreich's ataxia market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the Friedreich's ataxia market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the Friedreich's ataxia market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of Friedreich's ataxia across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Friedreich's ataxia by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Friedreich's ataxia by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with Friedreich's ataxia across the seven major markets?
- What is the size of the Friedreich's ataxia patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of Friedreich's ataxia?
- What will be the growth rate of patients across the seven major markets?
Friedreich's Ataxia: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Friedreich's ataxia drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Friedreich's ataxia market?
- What are the key regulatory events related to the Friedreich's ataxia market?
- What is the structure of clinical trial landscape by status related to the Friedreich's ataxia market?
- What is the structure of clinical trial landscape by phase related to the Friedreich's ataxia market?
- What is the structure of clinical trial landscape by route of administration related to the Friedreich's ataxia market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
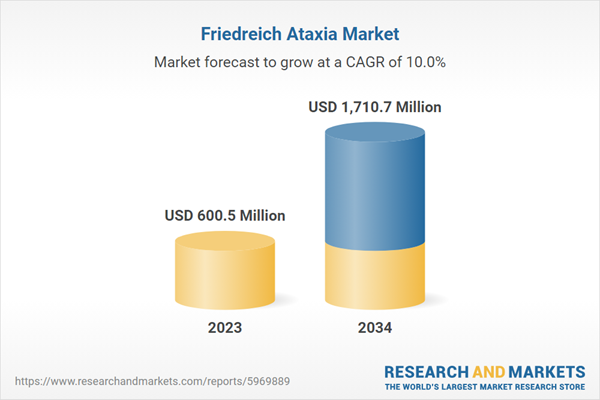
| Forecast Period | 2023 - 2034 |
| Estimated Market Value ( USD | $ 600.5 Million |
| Forecasted Market Value ( USD | $ 1710.7 Million |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |