The hidradenitis suppurativa market has been comprehensively analyzed in this report titled "Hidradenitis Suppurativa Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Hidradenitis suppurativa, also referred to as acne inversus, is a chronic, painful inflammatory skin condition that causes lumps to develop beneath the skin. These lumps generally form in areas where the skin rubs against itself, such as the groin, armpits, breasts, and buttocks. The condition tends to remain for an extended period and worsen over time, seriously affecting everyday life and emotional health. Individuals suffering from hidradenitis suppurativa may experience painful pea-sized lumps, leaking bumps or sores, blackheads, and tunnels under the skin. The other common symptoms include abscess, excessive sweating, body odor, itching, pimples, pus, swelling, etc. In severe cases, the disease can lead to significant discomfort, reduced mobility, and impaired quality of life. The diagnosis of this ailment is mainly based on medical history and the underlying symptoms. The healthcare professional may examine the affected skin region and sometimes test an infected part's sample. Additionally, various diagnostic procedures, such as ultrasound, magnetic resonance imaging (MRI), biopsy, etc., are further required to identify the extent of the disease.
The rising incidences of blocked hair follicles caused by several risk factors, including hormonal imbalances like polycystic ovarian syndrome, bacterial colonization, obesity, etc., are primarily driving the hidradenitis suppurativa market. Moreover, the increasing prevalence of genetic disorders that alter cell structure and cause immune dysfunction is further augmenting the market growth. Apart from this, the widespread adoption of antiandrogen therapy with spironolactone for treating the ailment, since it inhibits androgens from binding to androgen receptors and prevents cancer cells from growing, is acting as another growth-inducing factor. Furthermore, the inflating utilization of biologics, such as TNF-alpha inhibitors, which relieve disease symptoms by blocking the signaling pathway of a protein involved in the inflammatory process, is also bolstering the market growth. Additionally, several key players are making significant investments in developing imaging techniques that can help identify the extent and severity of the disease and can be particularly useful for assessing deep-seated lesions. This, in turn, is further creating a positive outlook for the market. Moreover, the escalating demand for advanced surgical procedures, such as cryoinsufflation, to effectively treat smaller lesions, especially those in areas where surgery may be difficult or for patients who cannot tolerate more invasive procedures, is also expected to bolster the hidradenitis suppurativa market in the coming years.
This report provides an exhaustive analysis of the hidradenitis suppurativa market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for hidradenitis suppurativa and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the hidradenitis suppurativa market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hidradenitis suppurativa market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hidradenitis suppurativa market
Competitive Landscape:
This report also provides a detailed analysis of the current hidradenitis suppurativa marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the hidradenitis suppurativa market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the hidradenitis suppurativa market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the hidradenitis suppurativa market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of hidradenitis suppurativa across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hidradenitis suppurativa by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of hidradenitis suppurativa by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with hidradenitis suppurativa across the seven major markets?
- What is the size of the hidradenitis suppurativa patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of hidradenitis suppurativa?
- What will be the growth rate of patients across the seven major markets?
Hidradenitis Suppurativa: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for hidradenitis suppurativa drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the hidradenitis suppurativa market?
- What are the key regulatory events related to the hidradenitis suppurativa market?
- What is the structure of clinical trial landscape by status related to the hidradenitis suppurativa market?
- What is the structure of clinical trial landscape by phase related to the hidradenitis suppurativa market?
- What is the structure of clinical trial landscape by route of administration related to the hidradenitis suppurativa market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 136 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
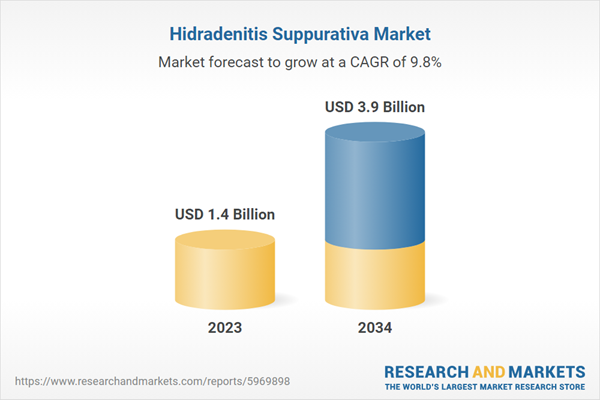
| Estimated Market Value ( USD | $ 1.4 Billion |
| Forecasted Market Value ( USD | $ 3.9 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |