The porphyria market has been comprehensively analyzed in this report titled "Porphyria Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Porphyria is a group of rare genetic disorders that result from abnormalities in the heme biosynthesis pathway, which leads to an accumulation of porphyrins and their precursors in the body. This ailment is mainly classified into acute and cutaneous porphyria. Acute porphyria is a short-term condition that primarily affects the nervous system and, in some cases, the skin. The usual symptoms are pain in the chest, abdomen, legs, or back, rapid or irregular heartbeats, seizures, mental changes, etc. Cutaneous porphyria is long-lasting and affects only the skin, leading to pain or blisters when the skin is exposed to sunlight. The symptoms mostly include changes in skin color or scarring, fragile skin, blisters on exposed skin, etc. The diagnosis of porphyria generally involves a combination of medical history, physical examination, and laboratory test procedures. The doctor might look for signs of skin sensitivity, abdominal pain, and muscle weakness during the physical examination. Laboratory tests to diagnose porphyria include measurement of porphyrin and precursor levels in the blood, urine, or stool samples. Additionally, genetic testing may also be used to identify specific enzyme deficiencies associated with different types of porphyria.
The increasing incidence of rare genetic disorders and the rising unmet medical need for effective therapies to treat such conditions are primarily driving the porphyria market. Additionally, the inflating usage of pain management medicines, including nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, nerve blocks, etc., to help alleviate the abdominal and neurological symptoms associated with the disease is also bolstering the market growth. Furthermore, the shifting preference from hematin injections towards panhematin infusions, since panhematin is easier to obtain, has a longer shelf life, and is less expensive than hematin, is acting as another significant growth-inducing factor. Besides this, the escalating utilization of erythropoietin injections to increase the production of red blood cells and heme in congenital erythropoietic porphyria patients is further creating a positive outlook for the market. Moreover, the widespread adoption of high-performance liquid chromatography (HPLC) to measure the levels of porphyrins and other heme precursors in blood, urine, and feces for the diagnosis of the ailment is also propelling the market growth. Apart from this, several biotechnological advancements, including the introduction of synthetic small interfering RNA (siRNA) molecules, which can decrease the buildup of neurotoxic porphyrin precursors and improve quality of life among patients, are expected to drive the porphyria market in the coming years.
This report provides an exhaustive analysis of the porphyria market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for porphyria and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the porphyria market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the porphyria market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the porphyria market
Competitive Landscape:
This report also provides a detailed analysis of the current porphyria marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the porphyria market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the porphyria market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the porphyria market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of porphyria across the seven major markets?
- What is the number of prevalent cases (2018-2034) of porphyria by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of porphyria by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of porphyria by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with porphyria across the seven major markets?
- What is the size of the porphyria patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of porphyria?
- What will be the growth rate of patients across the seven major markets?
Porphyria: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for porphyria drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the porphyria market?
- What are the key regulatory events related to the porphyria market?
- What is the structure of clinical trial landscape by status related to the porphyria market?
- What is the structure of clinical trial landscape by phase related to the porphyria market?
- What is the structure of clinical trial landscape by route of administration related to the porphyria market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 137 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
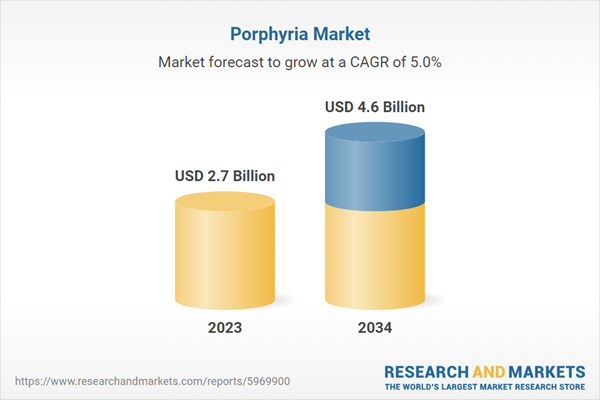
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 4.6 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |