The chronic hepatitis B market has been comprehensively analyzed in this report titled "Chronic Hepatitis B Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Chronic hepatitis B refers to a long-term viral infection caused by the hepatitis B virus (HBV) that affects the liver. It can manifest with a range of symptoms, although many individuals suffering from the ailment may remain asymptomatic for a long time. When indications do occur, they may include fatigue, nausea, abdominal discomfort, loss of appetite, jaundice (yellowing of the skin and eyes), etc. In some cases, the condition can progress to more advanced liver disease, leading to symptoms like fluid retention, easy bruising or bleeding, confusion, etc. The diagnosis of chronic hepatitis B involves a combination of blood tests and clinical evaluation. Serological tests are used to detect specific markers of the hepatitis B virus (HBV) infection, including hepatitis B e antigen (HBeAg), hepatitis B surface antigen (HBsAg), and antibodies against these antigens (anti-HBs and anti-HBe). HBV DNA testing is performed to measure the viral load in the blood. Additionally, various imaging studies, such as ultrasound and liver biopsy, may be conducted to evaluate liver structure and assess the degree of liver damage.
The increasing cases of unprotected sexual intercourse with an infected partner, sharing needles or other drug paraphernalia, coming into contact with infected blood or body fluids, etc., are primarily driving the chronic hepatitis B market. In addition to this, the widespread adoption of numerous antiviral agents, such as nucleotide/nucleoside analogs and pegylated interferon-alpha (PEG-IFN), for suppressing viral replication, decreasing liver inflammation, and slowing down the progression of the disease is also creating a positive outlook for the market. Moreover, the inflating application of liver fibrosis assessment methods, including transient elastography and serum biomarkers, since they offer a safer and less invasive alternative to liver biopsy, thereby reducing patient discomfort as well as risks associated with invasive procedures, is acting as another significant growth-inducing factor. Apart from this, the emerging popularity of immune modulation strategies, which stimulate the immune system while leading to sustained viral suppression and even serological response, such as hepatitis B surface antigen (HBsAg) loss, is expected to drive the chronic hepatitis B market during the forecast period.
This report provides an exhaustive analysis of the chronic hepatitis B market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for chronic hepatitis B and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the chronic hepatitis B market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the chronic hepatitis B market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the chronic hepatitis B market
Competitive Landscape:
This report also provides a detailed analysis of the current chronic hepatitis B marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the chronic hepatitis B market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the chronic hepatitis B market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the chronic hepatitis B market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of chronic hepatitis B across the seven major markets?
- What is the number of prevalent cases (2018-2034) of chronic hepatitis B by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of chronic hepatitis B by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with chronic hepatitis B across the seven major markets?
- What is the size of the chronic hepatitis B patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of chronic hepatitis B?
- What will be the growth rate of patients across the seven major markets?
Chronic Hepatitis B: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for chronic hepatitis B drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the chronic hepatitis B market?
- What are the key regulatory events related to the chronic hepatitis B market?
- What is the structure of clinical trial landscape by status related to the chronic hepatitis B market?
- What is the structure of clinical trial landscape by phase related to the chronic hepatitis B market?
- What is the structure of clinical trial landscape by route of administration related to the chronic hepatitis B market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
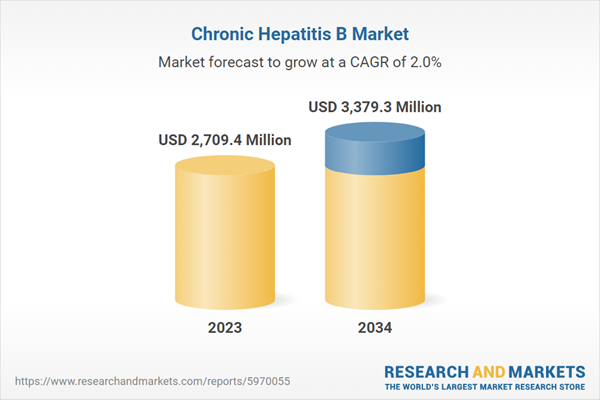
| Estimated Market Value ( USD | $ 2709.4 Million |
| Forecasted Market Value ( USD | $ 3379.3 Million |
| Compound Annual Growth Rate | 2.0% |
| Regions Covered | Global |