The ocular hypertension market has been comprehensively analyzed in this report titled "Ocular Hypertension Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Ocular hypertension refers to a condition characterized by higher-than-normal intraocular pressure (IOP) in the eye. Intraocular pressure is the fluid pressure inside the eye, maintained by the balance between the production and drainage of fluid called aqueous humor. Most people suffering from ocular hypertension are asymptomatic, but some patients may occasionally experience mild eye discomfort, including a feeling of pressure or aching in the eye. In rare cases, ocular hypertension may be accompanied by mild headaches, particularly around the temples or in the forehead. The diagnosis of ocular hypertension is typically made during a comprehensive eye examination. The main diagnostic criterion is the measurement of intraocular pressure using instruments like a tonometer. In general, an IOP reading of 21 mmHg or higher is considered elevated and indicative of ocular hypertension. The eye care professional may also assess the optic nerve through various techniques, such as ophthalmoscopy and optical coherence tomography (OCT). Additionally, a thorough examination of the anterior chamber angle may be performed to assess the drainage structures of the eye.
The escalating cases of inflammation, scarring, or structural abnormalities in the eye resulting from trauma or surgery, which can impair the proper outflow of aqueous humor, are primarily driving the ocular hypertension market. In addition to this, the rising prevalence of several associated risk factors, including genetic predisposition, age-related changes in the eye's drainage system, underlying systemic conditions like diabetes, prolonged use of corticosteroids, etc., is also bolstering the market growth. Furthermore, the inflating adoption of topical eye drops to lower elevated intraocular pressure by increasing the outflow of aqueous humor or reducing its production is acting as another significant growth-inducing factor. Additionally, the emerging popularity of cyclophotocoagulation, which uses laser or cryotherapy to target the ciliary body, thereby lowering its ability to produce aqueous humor, is also creating a positive outlook for the market. Apart from this, the escalating utilization of selective laser trabeculoplasty on account of its several associated benefits, such as targeted treatment without damaging surrounding tissues, minimal downtime and quick recovery, potential long-term advantages with sustained intraocular pressure control, etc., is expected to drive the ocular hypertension market in the coming years.
This report provides an exhaustive analysis of the ocular hypertension market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for ocular hypertension and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the ocular hypertension market in any manner.
Recent Developments:
In February 2024, Skye Bioscience, Inc. declared that it had dosed 56 patients with SBI-100 Ophthalmic Emulsion (OE) in its Phase 2a trial and completed final study visits for all patients. SBI-100 OE refers to a cannabinoid receptor type 1 agonist that is applied topically to the eye. It is being developed to meet the unmet requirements of patients with increased intraocular pressure caused by ocular hypertension.
In February 2024, Nicox SA announced the signing of an agreement that grants Kowa Company, Ltd. exclusive Japanese rights to develop and commercialize NCX 470, Nicox'sNicox's nitric oxide (NO)-donating bimatoprost eye drop, for the lowering of IOP in patients with ocular hypertension.
In December 2023, Glaukos reported that the FDA had approved a New Drug Application (NDA) for its iDose TR (travoprost intracameral) implant. This approval permits for iDose TR 75 mcg to be administered once per eye. The indication for the prostaglandin analog includes the decrease of IOP in individuals with ocular hypertension.
In September 2023, MERCURY-3 trial findings, which were published in the Graefe'sGraefe's Archive for Clinical and Experimental Ophthalmology, support the addition of Roclanda (latanoprost/netarsudil) as an alternative fixed-dose combination (FDC) treatment option for people affected with ocular hypertension.
Key Highlights:
The population prevalence estimates for ocular hypertension range from 4.5% to 9.4% for people older than 40 years, with frequency increasing with age.
African Caribbeans are more at risk for ocular hypertension, having the highest prevalence of the disease worldwide (12.6%).
In the United States, the incidence of ocular hypertension among non-Hispanic Whites aged 40 and above is 4.5 percent, rising to 7.7 percent in those aged 75 to 79.
According to research findings, around 10% of persons with untreated ocular hypertension develop primary open-angle glaucoma in five years.
Women have a higher risk of developing ocular hypertension than men.
Drugs:
Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% is a mixture of two prescription drugs that relieve high eye pressure (IOP) in people with open-angle glaucoma or ocular hypertension. The drug is recommended to be administered at a dosage of one drop once daily in the evening in the affected eye(s).
AGN-193408 sustained release is under clinical development by AbbVie for the treatment of ocular hypertension. This therapeutic candidate works by targeting the prostaglandin F2 alpha receptor and is administered as an implant via the ocular method.
QLS-101 is a new ATP-sensitive potassium (KATP) channel modulator delivered as a topical eyedrop. It addresses distal outflow resistance and episcleral venous pressure (EVP), which are important components of IOP. It also reduces IOP by expanding outflow channels and episcleral arteries in the eye distal to the trabecular meshwork.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the ocular hypertension market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the ocular hypertension market
Competitive Landscape:
This report also provides a detailed analysis of the current ocular hypertension marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the ocular hypertension market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the ocular hypertension market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the ocular hypertension market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of ocular hypertension across the seven major markets?
- What is the number of prevalent cases (2018-2034) of ocular hypertension by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of ocular hypertension by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with ocular hypertension across the seven major markets?
- What is the size of the ocular hypertension patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of ocular hypertension?
- What will be the growth rate of patients across the seven major markets?
Ocular Hypertension: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for ocular hypertension drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the ocular hypertension market?
- What are the key regulatory events related to the ocular hypertension market?
- What is the structure of clinical trial landscape by status related to the ocular hypertension market?
- What is the structure of clinical trial landscape by phase related to the ocular hypertension market?
- What is the structure of clinical trial landscape by route of administration related to the ocular hypertension market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
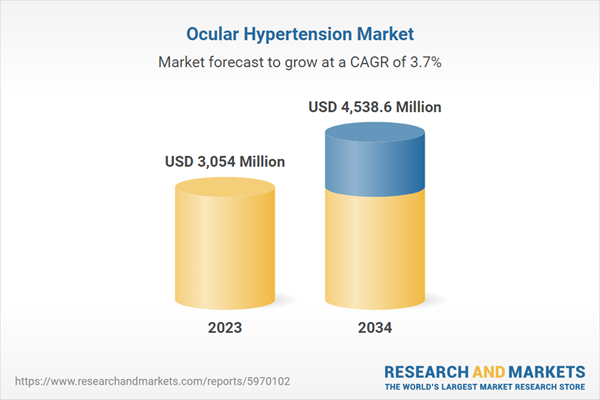
| Estimated Market Value ( USD | $ 3054 Million |
| Forecasted Market Value ( USD | $ 4538.6 Million |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |