The amebiasis market has been comprehensively analyzed in this report titled "Amebiasis Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Amebiasis is a gastrointestinal infection caused by the protozoan parasite Entamoeba histolytica. This condition is most prevalent in developing countries with poor sanitation conditions. It primarily affects the large intestine and is commonly transmitted through contaminated water or food. The symptoms of amebiasis can vary in severity, ranging from mild discomfort to life-threatening conditions. Common manifestations include abdominal pain, diarrhea (sometimes bloody), and cramping. In severe cases, the parasite can breach the intestinal wall, leading to the formation of abscesses in the liver or other organs, causing fever, fatigue, and potentially fatal complications. The diagnosis of amebiasis involves a combination of clinical assessment, stool sample analysis, and laboratory tests. A microscopic examination of stool samples is also recommended to identify the presence of infectious pathogens and diagnose the responsible species. The healthcare providers might further perform imaging studies like ultrasound or computed tomography (CT) scans to determine organ abscesses in individuals suffering from this disorder.
The escalating cases of parasitic infections, which spread through the consumption of food or water contaminated with fecal matter containing the parasite's cysts, are primarily driving the amebiasis market. In addition to this, the inflating utilization of efficacious medications, such as nitroimidazoles, luminal drugs, anti-amebic agents, etc., to manage symptoms and treat the illness is also creating a positive outlook for the market. Moreover, the widespread implementation of improved sanitation and hygiene practices, as they play a pivotal role in preventing the transmission of the causative microorganism and curtailing the spread of the disease, is further bolstering the market growth. Apart from this, the rising usage of supportive therapies and rehydration techniques, which aid in alleviating the indications and restoring electrolyte balance in individuals suffering from the disorder, is acting as another significant growth-inducing factor. Additionally, the emerging popularity of diagnostic techniques, such as molecular assays and serological tests, that can enable accurate and timely identification of the parasite, thereby boosting the quality of life for patients, is also augmenting the market growth. Furthermore, the increasing advancements in the development of vaccines for the underlying condition, since they involve the creation of formulations that confer immunity against E. histolytica infection, are expected to drive the amebiasis market during the forecast period.
This report provides an exhaustive analysis of the amebiasis market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for amebiasis and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the amebiasis market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the amebiasis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the amebiasis market
Competitive Landscape:
This report also provides a detailed analysis of the current amebiasis marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the amebiasis market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the amebiasis market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the amebiasis market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of amebiasis across the seven major markets?
- What is the number of prevalent cases (2018-2034) of amebiasis by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of amebiasis by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with amebiasis across the seven major markets?
- What is the size of the amebiasis patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of amebiasis?
- What will be the growth rate of patients across the seven major markets?
Amebiasis: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for amebiasis drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the amebiasis market?
- What are the key regulatory events related to the amebiasis market?
- What is the structure of clinical trial landscape by status related to the amebiasis market?
- What is the structure of clinical trial landscape by phase related to the amebiasis market?
- What is the structure of clinical trial landscape by route of administration related to the amebiasis market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 129 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
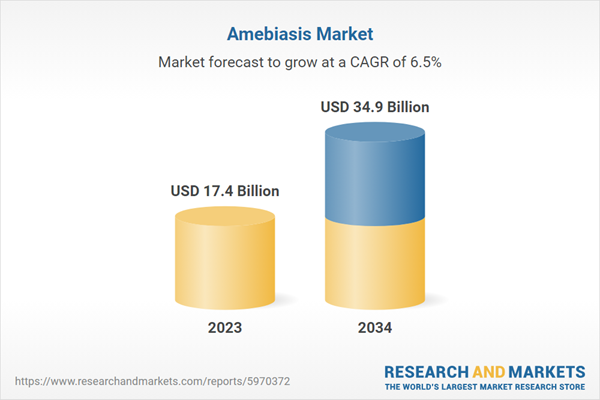
| Estimated Market Value ( USD | $ 17.4 Billion |
| Forecasted Market Value ( USD | $ 34.9 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |