Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Conversely, the market encounters substantial obstacles related to vaccine hesitancy and the sluggish uptake of recently authorized products, factors that jeopardize revenue growth. Skepticism surrounding new immunizations and insufficient public awareness frequently constrain the commercial reach of these biological treatments. For instance, the National Foundation for Infectious Diseases reported that in 2024, vaccination rates for respiratory syncytial virus among adults aged 60 and older in the United States stood at merely 24%, highlighting the difficulties in achieving widespread adoption.
Market Drivers
The Global RSV Drugs Market is being fundamentally transformed by recent regulatory clearances and the rollout of innovative vaccines. The introduction of targeted immunizations for seniors and long-acting monoclonal antibodies for infants has created a new financial foundation for the pharmaceutical industry. This transition from treating symptoms to preventative care enables companies to generate substantial value during peak viral seasons. As evidence of this success, Sanofi reported in its Q3 2024 results that global sales for the antibody Beyfortus hit €647 million in the third quarter alone, driven by robust seasonal demand and quick integration into immunization protocols, validating the industry's pivot toward preventative biologics.The significant burden of RSV-related hospital admissions serves as a crucial catalyst for market adoption, as healthcare systems strive to control costs linked to severe respiratory illness. The pressing clinical requirement to decrease admission rates among high-risk groups, such as neonates and the elderly, drives the utilization of these new therapeutics. The Centers for Disease Control and Prevention noted in a June 2024 report that RSV causes roughly 100,000 to 160,000 annual hospitalizations among US adults over 60, highlighting the vast patient population needing care. The economic magnitude of meeting this need is clear, with GSK reporting £1.2 billion in total sales for its Arexvy vaccine in its full-year 2023 results.
Market Challenges
Commercial expansion of the Global RSV Drugs Market is significantly hindered by vaccine hesitancy and the slow uptake of newly approved products. Although novel prophylactic and therapeutic options have been introduced, widespread skepticism and a lack of public knowledge severely limit the accessible patient population. This resistance to adopting biological agents results in lower sales volumes and a delayed return on investment for developers, as the theoretical market size does not translate into actual revenue as anticipated. Consequently, companies face the issue of unmoving inventory and the need for expensive, prolonged educational campaigns that fail to deliver immediate financial benefits.This pattern of weak market penetration is particularly noticeable in demographic segments where protection is most needed. For example, data from the Centers for Disease Control and Prevention indicated that maternal RSV vaccination coverage for the 2023-2024 season was only 32.6% in 2024. Such depressed immunization rates among priority groups clearly illustrate how hesitancy and sluggish adoption curb market growth. As a result, the industry confronts a flattened growth curve where the economic promise of advanced therapies is stifled by the enduring difficulty of securing end-user acceptance.
Market Trends
The sector is being revolutionized by the adoption of mRNA technology platforms for formulating novel RSV vaccines, facilitating the swift creation of highly effective immunizations. This technological advancement surpasses conventional protein subunit techniques by allowing for scalable manufacturing and precise antigen design targeting the prefusion F protein. The approval of the first mRNA-based RSV vaccine served as a validation of this innovation, setting a new standard for future biologics. In a May 2024 press release, Moderna announced that its mRESVIA vaccine received US FDA approval after demonstrating an efficacy of 83.7% against lower respiratory tract disease caused by RSV in adults aged 60 and older.Furthermore, the advancement of small molecule oral antiviral therapeutics in clinical pipelines marks a significant shift from strictly prophylactic approaches to acute disease management. Pharmaceutical companies are developing oral treatments to help infected patients, specifically addressing cases of vaccine breakthrough or high-risk individuals who remain unvaccinated. These innovative therapies are designed to drastically lower viral replication, thereby reducing symptom severity and halting transmission. Highlighting this progress, Enanta Pharmaceuticals reported positive topline results in September 2024 from a Phase 2a human challenge study, where their oral antiviral candidate EDP-323 achieved an 85% to 87% reduction in viral load area under the curve compared to a placebo.
Key Players Profiled in the Respiratory Syncytial Virus (RSV) Drugs Market
- AbbVie, Inc.
- AstraZeneca PLC
- Bausch Health Companies Inc.
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc.
- GlaxoSmithKline PLC
- Merck KgaA
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Johnson & Johnson Services, Inc.
Report Scope
In this report, the Global Respiratory Syncytial Virus (RSV) Drugs Market has been segmented into the following categories:Respiratory Syncytial Virus (RSV) Drugs Market, by Drug Type:
- Palivizumab
- Ribavirin
- Others
Respiratory Syncytial Virus (RSV) Drugs Market, by Dosage Form:
- Oral
- Injectable
- Others
Respiratory Syncytial Virus (RSV) Drugs Market, by Treatment Type:
- Antiviral Medications
- Immune prophylaxis
- Others
Respiratory Syncytial Virus (RSV) Drugs Market, by Distribution Channel:
- Hospital Pharmacies
- Specialty Pharmacies
- Online Pharmacies
Respiratory Syncytial Virus (RSV) Drugs Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Respiratory Syncytial Virus (RSV) Drugs Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Respiratory Syncytial Virus (RSV) Drugs market report include:- AbbVie, Inc.
- AstraZeneca PLC
- Bausch Health Companies Inc.
- F. Hoffmann-La Roche Ltd
- Gilead Sciences, Inc.
- GlaxoSmithKline PLC
- Merck KgaA
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Johnson & Johnson Services, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
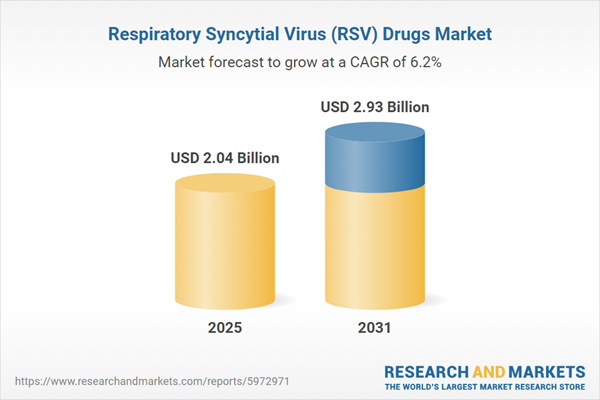
| Estimated Market Value ( USD | $ 2.04 Billion |
| Forecasted Market Value ( USD | $ 2.93 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |