Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive orientation to the saffron extract tablet landscape emphasizing clinical signals, consumer preferences, regulatory context, and commercial pathways
Saffron extract tablets are gaining heightened attention as clinical research, consumer wellness trends, and formulation science converge to create new commercial opportunities. This report frames the current landscape by synthesizing clinical signals around mood and inflammatory pathways, consumer-driven preferences for evidence-backed botanical products, and the evolving regulatory expectations that shape product positioning. Together, these forces are translating into differentiated product strategies, with some manufacturers prioritizing clinical substantiation while others emphasize clean-label sourcing and digital-first distribution.Across the value chain, there is a clear bifurcation between brands that invest in clinical validation and those that rely on traditional botanical heritage and marketing narratives. Meanwhile, supply-side considerations such as raw material traceability and extraction efficiencies are driving partnerships between ingredient suppliers and formulation houses. As a result, stakeholders need a multidimensional perspective that balances clinical credibility, supply resilience, and channel execution to translate saffron’s promise into commercial outcomes.
Key transformative shifts driven by extraction science, clinical validation, digital distribution, and regulatory tightening that are redefining market dynamics
Recent years have seen transformative shifts that are reshaping how saffron extract tablets are developed, positioned, and distributed. Advances in extraction methodology have increased purity and bioactive consistency, enabling smaller, more potent doses that support convenient tablet formats. At the same time, clinical research programs that target mood regulation and inflammatory biomarkers are delivering data that manufacturers can use to support differentiated claims, creating a new tier of clinically oriented botanical products.Concurrently, digital commerce and direct-to-consumer marketing have accelerated consumer access to niche formulations and facilitated rapid feedback loops that inform iterative product development. Regulatory scrutiny has intensified in parallel, prompting clearer labeling and substantiation expectations across major markets. Taken together, these developments are elevating the importance of integrated strategies that synchronize R&D, regulatory affairs, and commercial execution to capture emerging consumer demand and defend against competitive erosion.
How the United States tariff adjustments implemented in 2025 created supply chain, sourcing, and pricing ripple effects across saffron tablet value chains
Policy shifts in tariff regimes can have multifaceted effects across ingredient sourcing, manufacturing economics, and channel pricing, and the changes enacted in the United States in 2025 exemplify this complexity. Import duties and classification adjustments affecting botanical extracts altered the relative cost structure for raw saffron and processed extracts, prompting firms to revisit supplier contracts, reoptimize manufacturing footprints, and, in some instances, accelerate vertical integration to secure margin stability. These responses have been uneven: manufacturers with nearshore or domestic extraction capabilities absorbed shocks more efficiently, whereas those dependent on distant suppliers faced longer lead times and higher landed costs.The tariff environment also reshaped commercial conversations between buyers and suppliers, increasing the emphasis on cost-of-goods transparency and long-term supply agreements. Retail pricing strategies and promotional cadence shifted in response to the altered cost basis, and private-label manufacturers in particular reassessed SKU portfolios to prioritize higher-margin formulations. In short, the cumulative impact of the 2025 tariff adjustments has been to elevate supply chain resilience, supplier diversification, and cost engineering as strategic priorities for companies engaged in saffron tablet commercialization.
In-depth segmentation analysis revealing how channels, product formulations, dosages, applications, end users, gender, and age cohorts shape strategic opportunities
A nuanced segmentation framework illuminates where demand concentration, formulation innovation, and channel economics converge to create distinct strategic priorities. Distribution channel dynamics reveal a split between institutional procurement and direct consumer access: hospitals and retail pharmacies continue to play critical roles in therapeutic positioning, while online pharmacies-both manufacturer websites and broader marketplaces-enable targeted consumer engagement and subscription models that favor convenience and replenishment. Product type segmentation uncovers divergent trajectories between single-ingredient saffron formulations designed for focused clinical claims and combination offerings that blend saffron with herbal or vitamin complements; the latter, whether configured as herbal combinations or vitamin combinations, are often engineered to broaden appeal or support multifunctional claims.Dosage strength considerations further stratify SKU planning, with high dose, medium dose, and low dose options supporting different clinical intentions and consumer tolerances. Application categories separate mainstream dietary supplement positioning from therapeutic use cases in which anti-depressant or anti-inflammatory intents command more rigorous evidence and clinical engagement. End user distinctions between general consumers and healthcare professionals-where the latter group includes nurses and physicians-influence messaging, channel choice, and educational investments. Finally, demographic segments based on gender and age-female and male cohorts, along with adults and senior citizens-drive tailored formulations and communication strategies that reflect differing health priorities and consumption patterns. Together, these segmentation lenses inform portfolio decisions, marketing segmentation, and clinical investment.
Regional dynamics across the Americas Europe Middle East & Africa and Asia-Pacific that determine sourcing priorities regulatory approaches and distribution strategies
Regional dynamics exert a strong influence on sourcing strategies, regulatory compliance, distribution pathways, and consumer acceptance. In the Americas, market activity is shaped by robust retail and e-commerce infrastructures, a high degree of consumer interest in botanical wellness, and regulatory frameworks that emphasize labeling and claim substantiation. Actors operating in this region often prioritize clinical studies and strong chain-of-custody documentation to meet both retailer and healthcare stakeholder expectations. In Europe, Middle East & Africa, the regulatory landscape is heterogeneous, with some jurisdictions demanding pharmacy-level oversight for therapeutic positioning while others enable wider supplement distribution; this diversity requires adaptive regulatory strategies and localized safety dossiers.In Asia-Pacific, supply chain proximity to key saffron-producing regions and established botanical manufacturing capabilities present both opportunities and competitive pressures. Regional consumer preferences and traditional uses of saffron inform product storytelling and formulation choices, while emerging e-commerce ecosystems support rapid scaling. Across all regions, cross-border regulatory harmonization remains partial, so manufacturers must reconcile global branding ambitions with local compliance nuances and distribution realities.
Competitive landscape and company strategies that emphasize clinical evidence supply chain control formulation partnerships and omnichannel commercialization tactics
Companies competing in the saffron tablet segment are differentiating along several dimensions including evidence generation, supply chain control, formulation innovation, and channel orchestration. Market leaders tend to allocate resources toward clinical programs and peer-reviewed publications that substantiate therapeutic claims, thereby gaining access to healthcare professional endorsement and specialty channels. Concurrently, some players invest in upstream integration-securing contracting relationships with growers or establishing proprietary extraction facilities-to guarantee consistent bioactive profiles and traceability.On the commercial front, successful firms leverage omnichannel strategies that blend direct-to-consumer platforms, marketplace presence, and traditional pharmacy relationships to optimize reach and margins. Innovation partnerships between ingredient specialists and nutraceutical formulators have become common, enabling rapid iteration of combination products and targeted dosage strengths. At the same time, smaller, agile brands use storytelling and influencer-driven education to capture niche audiences, while more established firms rely on the credibility that comes with clinical partnerships and institutional endorsements. The interplay between these strategic choices determines competitive positioning and resilience in the face of regulatory or supply disruptions.
Actionable strategic recommendations for manufacturers and brand leaders to strengthen evidence generation supply resilience channel mix and regulatory preparedness
Industry leaders should pursue a coordinated set of actions that reinforce product credibility, supply resilience, and channel effectiveness. First, prioritize investment in targeted clinical programs that align with the most commercially salient therapeutic endpoints; robust, peer-reviewed evidence will unlock access to professional channels and support premium positioning. Second, diversify supplier relationships and consider strategic vertical integration for critical extraction or processing capabilities to mitigate tariff exposure and strengthen traceability.Third, adopt differentiated channel strategies that combine direct-to-consumer subscription models through manufacturer platforms with curated marketplace presence and selective pharmacy placements to balance reach and margin. Fourth, design product portfolios that leverage dosage tiering and combination formulations to meet segmented consumer needs while simplifying manufacturing complexity. Fifth, develop a regulatory playbook that anticipates cross-jurisdictional claim requirements and invests in labeling and safety documentation early in the product development cycle. Implementing these recommendations in an integrated manner will enhance commercial agility and reduce exposure to supply and policy shocks.
Robust mixed-methods research methodology combining primary stakeholder interviews secondary literature synthesis and cross-validation to ensure analytical rigor and transparency
The analysis underpinning this report uses a mixed-methods approach that integrates primary stakeholder engagement with comprehensive secondary research. Primary inputs include structured interviews with formulation scientists, regulatory specialists, procurement leads, and commercial executives across distribution channels, providing firsthand insights into operational constraints, clinical priorities, and go-to-market tactics. These qualitative perspectives were triangulated with targeted quantitative data points drawn from trade publications, product labeling reviews, and regulatory filings to validate thematic conclusions.Data integrity was further reinforced through cross-validation exercises with supply chain partners and clinical research sponsors, and through examination of manufacturing and extraction process literature. Limitations are acknowledged: certain proprietary commercial data remain subject to confidentiality constraints, and regulatory interpretation can vary by jurisdiction. To address these factors, the methodology emphasizes transparency in source provenance, conservative interpretation of forward-looking statements, and a focus on actionable intelligence rather than speculative market projections.
Conclusive synthesis emphasizing the interplay of clinical validation sourcing resilience regulatory complexity and channel strategy as determinants of future success
In synthesis, the saffron extract tablet market is at an inflection point where scientific validation, supply chain discipline, and digital commercialization converge to determine competitive advantage. Companies that align product development with credible clinical evidence while securing resilient sourcing and adopting nimble channel strategies will be best positioned to capture emerging opportunities. Regulatory complexity across regions compels adaptive compliance strategies, and tariff-driven cost dynamics emphasize the need for supply portfolio diversification and cost transparency.Looking ahead, the interplay between innovation in extraction and formulation, the accumulation of clinical data, and evolving consumer preferences for vetted botanical solutions will shape which products gain mainstream acceptance. Stakeholders who act decisively on evidence generation, invest in supply chain robustness, and tailor distribution models to segmented demand will increase their odds of sustainable success in this rapidly evolving category.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Saffron Extract Tablet Market
Companies Mentioned
The key companies profiled in this Saffron Extract Tablet market report include:- Ayush Herbs, Inc.
- Bio Nutrition Inc.
- Hard Eight Nutrition LLC
- Lallemand Inc.
- Life Extension Foundation Buyers Club, Inc.
- Luma Nutrition
- NOW Health Group, Inc.
- Nutricost
- Nutritional Products Ltd
- Persavita, Inc
- Sunday Natural Products GmbH
- Swanson Health Products, Inc.
- The Naked Pharmacy Ltd.
- Vox Nutrition Inc.
- Zyrex Healthcare Pvt Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
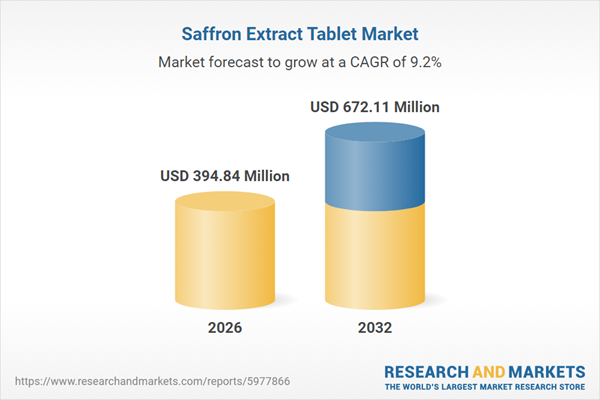
| Estimated Market Value ( USD | $ 394.84 Million |
| Forecasted Market Value ( USD | $ 672.11 Million |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |