Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive developments, the market encounters significant hurdles regarding production scalability and supply chain complexity. The personalized nature of these treatments necessitates a rigorous "vein-to-vein" process that is both labor-intensive and logistically demanding. Consequently, high manufacturing costs remain a substantial challenge, potentially hindering the widespread adoption and expansion of autologous cell therapies across global healthcare systems.
Market Drivers
The rising global prevalence of cancer and autoimmune disorders acts as the primary engine for the autologous cell therapy market, creating an urgent need for treatments capable of addressing refractory conditions. As the limitations of standard care become evident in complex indications such as multiple myeloma, the demand for personalized cellular interventions is increasing sharply. This growing disease burden is translating directly into significant therapy adoption and commercial volume; for instance, Legend Biotech’s "Fourth Quarter and Full Year 2024 Financial Results" in March 2025 reported that its autologous CAR-T therapy, Carvykti, generated $963 million in full-year sales and treated over 5,000 patients globally, demonstrating how persistent oncological diagnoses are driving the integration of these advanced modalities into routine clinical practice.Simultaneously, a surge in regulatory approvals for CAR-T and gene-modified therapies is accelerating market maturity. Regulatory bodies are increasingly clearing novel autologous products for earlier lines of therapy, thereby validating their safety profiles and opening new revenue streams. This momentum is reflected in the growing volume of approved therapies entering the commercial space; according to the Alliance for Regenerative Medicine’s "State of the Industry Briefing" in January 2025, the sector secured nine regulatory approvals in 2024, signaling a robust shift from clinical development to market availability. This regulatory success is fueling rapid financial growth, as evidenced by Bristol Myers Squibb’s February 2025 report, which noted that global revenue for its cell therapy Breyanzi reached $747 million, marking a 105% increase compared to the previous year.
Market Challenges
The high cost of manufacturing, driven by limited scalability and supply chain complexity, presents a formidable barrier to the growth of the Global Autologous Cell Therapy Market. Unlike traditional pharmaceutical or allogeneic treatments, autologous therapies rely on a patient's own cells, necessitating a bespoke, labor-intensive manufacturing process for each individual dose. This lack of standardization prevents the industry from achieving economies of scale, resulting in exorbitant costs of goods sold that restrict reimbursement potential and limit patient access within global healthcare systems.This production bottleneck is becoming increasingly critical as the volume of developing treatments grows. According to the International Society for Cell & Gene Therapy, the global pipeline expanded in 2024 to include 3,063 active therapies, representing a 7.5 percent increase in the first half of the year alone. This rapid accumulation of clinical candidates places immense pressure on an already strained manufacturing infrastructure. Without the capacity to efficiently scale production to meet this rising demand, the market faces a "commercialization bottleneck" where innovative therapies are approved but cannot be delivered at a viable price point or volume.
Market Trends
The expansion of autologous CAR-T therapies into autoimmune indications marks a fundamental market pivot, extending beyond oncology to treat severe conditions such as systemic lupus erythematosus and multiple sclerosis. Developers are harnessing the ability of engineered T-cells to deeply deplete B-cells and reset the immune system, offering a potential functional cure for patients refractory to standard immunosuppressants. This intensifying clinical focus is rapidly building a robust development pipeline that diversifies the sector's reliance on cancer treatments; for example, a July 2024 Medscape article titled "CAR T-Cell Studies in Autoimmune Diseases Are Proliferating" noted approximately 40 active studies utilizing CAR-T cells across various autoimmune indications, reflecting a significant resource shift toward this new therapeutic frontier.Concurrently, the commercialization of Tumor-Infiltrating Lymphocyte (TIL) therapies represents a critical breakthrough in the solid tumor segment, effectively widening the addressable market beyond hematological malignancies. This trend involves extracting and invigorating patient-specific immune cells to target complex cancer microenvironments in indications like metastatic melanoma, which have historically been difficult to treat with cellular modalities. The immediate market traction of these novel therapies underscores their commercial viability; according to Iovance Biotherapeutics’ "Third Quarter and Year to Date 2024 Financial Results" from November 2024, the company’s TIL therapy Amtagvi generated $42.1 million in U.S. net product revenue during the third quarter, signaling a strong adoption trajectory for solid tumor cell therapies.
Key Players Profiled in the Autologous Cell Therapy Market
- Biomatrica, Inc.
- Holostem Terapie Avanzate S.R.L
- Pharmicell Co. Inc.
- Caladrius Biosciences Inc.
- U.S. Stem Cell Inc.
- Bristol Myers Squibb Company
- Corning Incorporated
- Vericel Corporation
- Catalent, Inc.
- Sartorius AG
Report Scope
In this report, the Global Autologous Cell Therapy Market has been segmented into the following categories:Autologous Cell Therapy Market, by Source:
- Bone Marrow
- Epidermis
- Mesenchymal Stem Cells
- Haematopoietic Stem Cells
- Chondrocytes
- Others
Autologous Cell Therapy Market, by Application:
- Cancer
- Neurodegenerative Disorders
- Cardiovascular Disorders
- Autoimmune Disorders
- Orthopedics
- Wound Healing
- Others
Autologous Cell Therapy Market, by End User:
- Hospitals & Clinics
- Ambulatory Centers
- Others
Autologous Cell Therapy Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Autologous Cell Therapy Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Autologous Cell Therapy market report include:- Biomatrica, Inc.
- Holostem Terapie Avanzate S.R.L
- Pharmicell Co. Inc
- Caladrius Biosciences Inc
- U.S. Stem Cell Inc
- Bristol Myers Squibb Company
- Corning Incorporated
- Vericel Corporation
- Catalent, Inc
- Sartorius AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
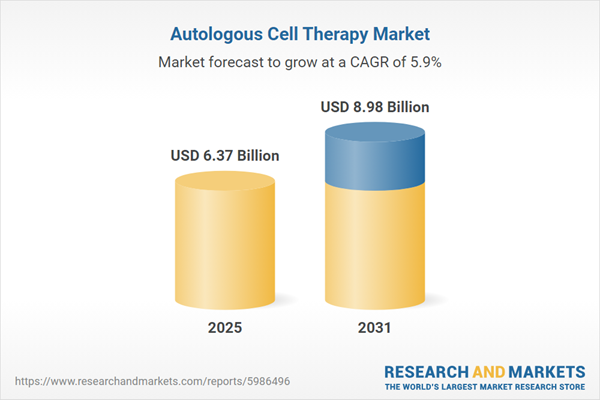
| Estimated Market Value ( USD | $ 6.37 Billion |
| Forecasted Market Value ( USD | $ 8.98 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |