Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Recent developmental data highlights the sector's vitality; as noted by the American Society of Gene & Cell Therapy in 2024, the global therapy pipeline encompassed more than 4,000 treatments, with gene therapies accounting for nearly 50% of these candidates. Despite this progressive momentum, the industry faces significant hurdles related to the high complexity and expense of large-scale vector manufacturing. These production challenges remain a substantial obstacle that threatens to hinder widespread market adoption and limit commercial scalability.
Market Drivers
Significant technical breakthroughs in viral and non-viral vector systems are driving the market forward by transforming experimental candidates into commercially available treatments. The sector has reached a crucial stage of maturity, progressing from initial proof-of-concept phases to achieving regulatory success for complex modalities like advanced adeno-associated virus vectors and CRISPR-based editing. This evolution is highlighted by a record surge in market activity; the Alliance for Regenerative Medicine reported in January 2024 that the US FDA approved seven gene therapies in 2023, marking the highest annual total to date. These approvals confirm the effectiveness of next-generation delivery platforms and encourage sustained investment in novel programmable therapies, as evidenced by Arsenal Biosciences raising $325 million in Series C financing in September 2024 to advance its programmable cell therapy programs for solid tumors.Furthermore, a rise in strategic partnerships and industry collaborations is serving as a key catalyst for growth, allowing firms to manage high development expenses and manufacturing difficulties. Biopharmaceutical leaders are actively seeking mergers and licensing deals to incorporate proprietary capsid technologies and expedite clinical pipelines without bearing the entire weight of internal research. Such alliances are critical for scaling up production and obtaining the specialized delivery mechanisms necessary for global commercialization. A notable example of this trend occurred in October 2024, when Astellas Pharma finalized an exclusive option and license agreement with AviadoBio, potentially worth up to $2.18 billion, to develop gene therapies targeting frontotemporal dementia.
Market Challenges
The substantial cost and complexity inherent in large-scale vector manufacturing present a major constraint on the Global Gene Delivery Technologies Market. In contrast to traditional pharmaceutical manufacturing, the creation of viral vectors requires intricate biological processes that are intrinsically challenging to standardize and scale. This limitation in manufacturing scalability creates significant supply chain bottlenecks and leads to a higher Cost of Goods Sold (COGS). As manufacturers encounter difficulties in efficiently producing commercial quantities, the resulting financial burden is frequently transferred to the healthcare system, resulting in restricted insurance coverage and diminished market penetration.This difficulty is quantitatively evident in the pricing strategies for recently authorized treatments. Data from the Alliance for Regenerative Medicine in 2024 indicates that the wholesale acquisition cost for a single gene therapy treatment has climbed to approximately $3.1 million. Such elevated price points, driven by the capital-intensive requirements of vector production, severely limit the patient population capable of accessing these life-changing therapies. Consequently, the market's full commercial potential remains unrealized, as prohibitive manufacturing costs hinder the ability of these therapies to reach a wider audience.
Market Trends
The incorporation of Artificial Intelligence into vector design is revolutionizing the discovery and refinement of gene delivery vehicles. By utilizing machine learning algorithms, researchers are shifting away from traditional empirical screening methods toward the computational engineering of synthetic capsids that offer improved tissue tropism and lowered immunogenicity.This data-centric strategy enables the simulation of intricate biological interactions, vastly speeding up the identification of viable candidates capable of evading pre-existing immunity while optimizing transduction efficiency. The significance of this technological evolution is demonstrated by the strategies of key industry players; for instance, Inc. Magazine reported in May 2025 that Moderna has implemented over 3,000 customized artificial intelligence models across its operations to enhance the design and development of mRNA medicines and their delivery systems.
Concurrently, the market is witnessing an accelerated shift toward Lipid Nanoparticles (LNPs) as the preferred non-viral delivery method for an expanding array of therapeutic payloads. Although viral vectors have traditionally held dominance, the superior scalability, safety profile, and versatility of LNPs are fueling their growth beyond vaccine applications into complex protein replacement and gene editing therapies. Leading pharmaceutical companies are aggressively acquiring proprietary LNP platforms to ensure robust delivery capabilities for their genetic medicine pipelines, thereby circumventing the manufacturing bottlenecks often linked to viral vectors. This trend of strategic consolidation is exemplified by recent high-value deals, such as AbbVie's agreement in June 2025 to acquire Capstan Therapeutics for up to $2.1 billion to integrate its targeted lipid nanoparticle technology for in vivo cell reprogramming.
Key Players Profiled in the Gene Delivery Technologies Market
- Thermo Fisher Scientific, Inc.
- Promega Corporation
- Qiagen N.V.
- Horizon Discovery Ltd.
- OriGene Technologies, Inc.
- Oxford Biomedica PLC
- SignaGen Laboratories
- Takara Bio Inc.
- Bio-Rad Laboratories, Inc.
- System Biosciences, LLC
Report Scope
In this report, the Global Gene Delivery Technologies Market has been segmented into the following categories:Gene Delivery Technologies Market, by Mode:
- Biological
- Chemical
- Physical
Gene Delivery Technologies Market, by Method:
- Ex vivo
- In vivo
- In vitro
Gene Delivery Technologies Market, by Application:
- Gene Therapy
- Cell Therapy
- Vaccines
- Research
Gene Delivery Technologies Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Gene Delivery Technologies Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Gene Delivery Technologies market report include:- Thermo Fisher Scientific, Inc
- Promega Corporation
- Qiagen N.V.
- Horizon Discovery Ltd.
- OriGene Technologies, Inc.
- Oxford Biomedica PLC
- SignaGen Laboratories
- Takara Bio Inc.
- Bio-Rad Laboratories, Inc.
- System Biosciences, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
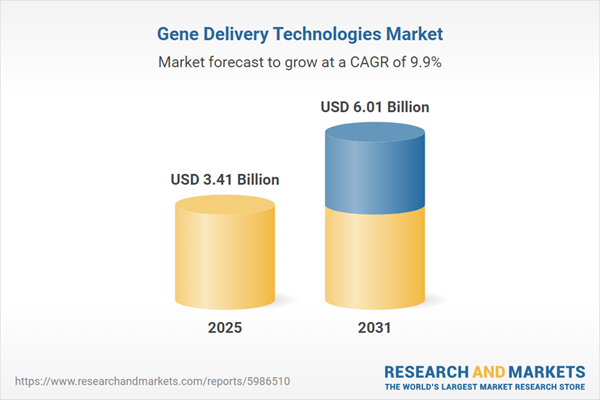
| Estimated Market Value ( USD | $ 3.41 Billion |
| Forecasted Market Value ( USD | $ 6.01 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |