Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these advancements, significant barriers exist regarding the substantial capital and operating costs needed for cold chain infrastructure, limiting rapid scalability. The burden of these expenses is magnified by the increasing quantity of therapeutic candidates requiring reliable preservation during development. Data from the Alliance for Regenerative Medicine indicates that 2,406 clinical trials for cell and gene therapies were active globally in 2024. Although this high volume suggests robust demand, the financial intricacies involved in storing such a large amount of biological material continue to impede efficient supply chain management.
Market Drivers
The rapid growth of the regenerative medicine and cell therapy sectors propels the market by demanding strong cold chain infrastructure. As gene-edited treatments move from clinical trials to the commercial market, there is a growing need for decentralized preservation facilities, a trend bolstered by significant industry investment aimed at speeding up pipeline development. The Alliance for Regenerative Medicine reported in October 2024 that the sector secured $10.9 billion in the first half of the year. Consequently, companies are building vast treatment networks; for instance, Vertex Pharmaceuticals noted in February 2025 that it had established over 50 authorized treatment centers worldwide by the end of 2024, highlighting the reliance on cryopreservation for maintaining autologous product viability.Additionally, the rising prevalence of infertility and the demand for Assisted Reproductive Technologies (ART) significantly boost market growth, as modern fertility procedures rely heavily on the vitrification of biological samples. There is a notable industry shift toward "freeze-all" strategies and elective fertility preservation, necessitating long-term storage capabilities. In April 2025, the Society for Assisted Reproductive Technology reported that member clinics performed 432,641 IVF cycles in 2023. This increase in procedure volume directly drives the need for sophisticated cryopreservation media and automated storage solutions to preserve gametes and embryos over long periods.
Market Challenges
The substantial capital and operational costs required to build and sustain cold chain infrastructure represent a major obstacle to the growth of the cell cryopreservation market. Setting up reliable storage facilities demands heavy upfront investment in specialized freezers, automated monitoring, and backup power systems to guarantee sample safety. Beyond these initial expenditures, ongoing costs for liquid nitrogen, equipment upkeep, and adherence to strict regulations impose a continuous financial burden on preservation providers and biopharmaceutical developers.This economic strain is worsened by the massive volume of biological materials needing secure, long-term storage throughout the development process. As the therapeutic pipeline grows, the total cost of preserving these delicate materials rises, often straining the budgets of smaller development companies. The American Society of Gene & Cell Therapy noted in 2024 that the global pipeline contained 4,021 gene, cell, and RNA therapies across various stages. Managing the storage logistics for such a vast array of developmental assets necessitates significant capital, effectively limiting the market's ability to scale operations efficiently.
Market Trends
Biobanking workflows are increasingly incorporating robotic automation to transform sample management, reducing human contact and ensuring stable temperatures during handling. To enhance efficiency and decrease errors, facilities are adopting automated retrieval systems that keep samples at ultra-low temperatures without the fluctuations caused by manual access. This trend is reflected in rising investments in advanced management infrastructure; Azenta Life Sciences reported in May 2025 that its Sample Management Solutions revenue hit $80 million in the second quarter of fiscal 2025, an 8% rise driven by strong demand for automated storage and clinical sample services.Concurrently, there is a significant shift toward using ready-to-use, GMP-compliant cryopreservation media to satisfy rigorous regulatory requirements for cell and gene therapies. Developers are abandoning traditional serum-based or homemade formulations in favor of chemically defined options that provide better consistency between lots and lower toxicity risks. The uptake of these optimized tools is speeding up as commercial therapies expand manufacturing; BioLife Solutions announced in May 2025 that its cell processing revenue grew 33% year-over-year to $21.6 million, largely due to the integration of its GMP-grade media into the production of 17 approved commercial therapies.
Key Players Profiled in the Cell Cryopreservation Market
- Thermo Fisher Scientific Inc.
- PromoCell GmbH
- Merck KGaA
- Sartorius AG
- Lonza Group Ltd.
- Corning Incorporated
- Creative Biolabs
- BioLife Solutions Inc.
- Danaher Corporation
- HiMedia Laboratories Pvt. Ltd.
Report Scope
In this report, the Global Cell Cryopreservation Market has been segmented into the following categories:Cell Cryopreservation Market, by Product:
- Cell Freezing Media
- Equipment
- Consumables
Cell Cryopreservation Market, by Application:
- Stem Cells
- Oocytes and Embryotic Cells
- Sperm Cells
- Hepatocytes
- Others
Cell Cryopreservation Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cell Cryopreservation Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cell Cryopreservation market report include:- Thermo Fisher Scientific Inc.
- PromoCell GmbH
- Merck KGaA
- Sartorius AG
- Lonza Group Ltd.
- Corning Incorporated
- Creative Biolabs
- BioLife Solutions Inc.
- Danaher Corporation
- HiMedia Laboratories Pvt. Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
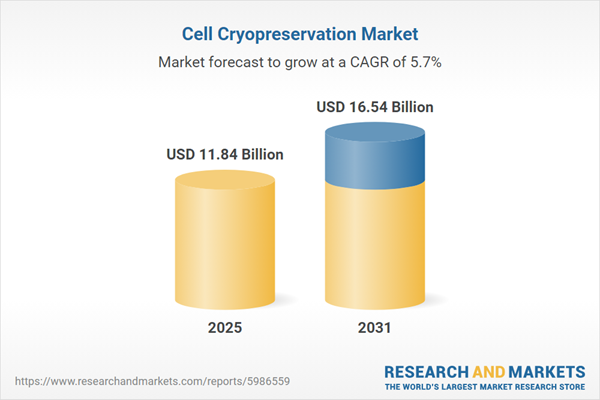
| Estimated Market Value ( USD | $ 11.84 Billion |
| Forecasted Market Value ( USD | $ 16.54 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |