Spinal Implants Market
The spinal implants market encompasses a wide range of devices and adjuncts used to stabilize, correct, and preserve motion across cervical, thoracic, and lumbar indications. Core applications include degenerative disc disease, deformity correction, trauma and fracture management, tumor-related reconstruction, and revision procedures. End-use settings span tertiary hospitals, specialty spine centers, and increasingly ambulatory surgery centers for select minimally invasive lumbar and cervical cases. The product universe covers pedicle screw systems, interbody cages and spacers, fixation plates, hooks and rods, artificial discs and other motion-preserving devices, vertebral body replacement systems, and biologics that support fusion. Recent trends emphasize minimally invasive approaches paired with intraoperative navigation, robotics, and advanced imaging; patient-specific planning and 3D-printed porous architectures that optimize osseointegration; expandable interbody designs to restore alignment; and growing interest in motion preservation where clinically appropriate. Key demand drivers include an aging population with multi-level degeneration, a widening pool of trained spine surgeons, and continual improvements in implant materials, surface technologies, and surgical workflows that reduce operative time and length of stay. The competitive landscape features diversified global medtech leaders and focused spine specialists, each differentiating through complete procedure solutions, surgeon education, digital planning tools, and service models aligned with value-based care. Reimbursement clarity in mature markets, public procurement in cost-sensitive regions, and evolving regulatory requirements shape go-to-market strategies. Overall, the market is shifting from standalone implants to integrated ecosystems that combine hardware, enabling technologies, preoperative planning, and data services to deliver reproducible outcomes and economic value for providers.Spinal Implants Market Key Insights
- Procedure mix: fusion remains foundational, with nuanced shifts. Posterior and lateral fusion techniques continue to anchor volumes across degenerative and deformity care. Surgeons balance interbody options by level and pathology, while vertebral body replacement grows in complex oncology and trauma. Motion preservation expands selectively where facet health and alignment permit durable outcomes.
- Minimally invasive surgery (MIS) broadens across levels. Tubular and percutaneous access reduces blood loss and recovery times, supporting same-day or short-stay pathways. Lateral and oblique corridors enable larger graft footprints with smaller incisions. Vendors that pair implants with MIS instruments, retractors, and education programs see stronger pull-through.
- Navigation, robotics, and imaging drive accuracy and efficiency. Optical and 3D navigation, robotic guidance, and intraoperative CT improve screw placement and reproducibility. The business case strengthens when platforms integrate preoperative planning, implant libraries, and analytics, shortening learning curves and standardizing OR workflows.
- Interbody innovation centers on surface and structure. Porous titanium, additive-manufactured lattices, and roughened or bioactive surfaces enhance mechanical interlock and bone response. Expandable devices aid disc height and lordosis restoration while reducing endplate preparation demands, though surgeons weigh subsidence risks and cost.
- Biologics remain a pivotal yet scrutinized adjunct. Demineralized matrices, synthetics, cell-based options, and growth-factor substitutes aim to improve fusion quality and timelines. Choice is driven by indication, comorbidity profile, and payer policy, with hospitals seeking predictable performance and carefully managed costs.
- Motion preservation targets defined patient cohorts. Cervical disc arthroplasty gains traction on kinematics and adjacent-segment considerations. Lumbar disc solutions show selective use where patient selection, implant design, and surgeon expertise align. Long-term evidence and reimbursement pathways shape adoption pace.
- Personalization and 3D printing elevate fit and planning. Patient-specific guides, custom interbodies, and tailored alignment plans improve endplate conformity and load sharing. Advanced planning software links imaging to implant selection, supporting consistent correction targets in deformity and complex revisions.
- Site-of-care shift and economics reframe value. Ambulatory and short-stay pathways favor MIS constructs, streamlined trays, and vendor support that reduces sterilization and inventory burdens. Bundled payments and episode-based models reward fewer complications, lower readmissions, and efficient implant logistics.
- Regulatory rigor and quality management intensify. Heightened post-market surveillance, traceability, and material documentation influence design choices and supplier selection. Companies invest in risk management, human-factors engineering, and complaint handling to sustain approvals and tender eligibility.
- Commercial models evolve with integrated ecosystems. Portfolio breadth, capital-light access to enabling tech, and robust surgeon training are decisive. Partnerships around robotics, imaging, and data analytics, plus responsive field service and hybrid consignment, strengthen competitive stickiness at the hospital level.
Spinal Implants Market Reginal Analysis
North America
Adoption of MIS, navigation, and robotic guidance is widespread, underpinned by a dense network of fellowship-trained surgeons and technology-capable facilities. Reimbursement frameworks increasingly evaluate total episode costs, encouraging standardized trays, reduced biologics variability, and predictable outcomes. Ambulatory surgery centers are expanding indications for select cervical and single-level lumbar procedures, catalyzing demand for streamlined systems. Provider consolidation increases purchasing leverage, prompting vendors to deliver bundled solutions and training. Data-driven decision support and infection-prevention protocols remain central to value demonstration.Europe
Aging demographics and high clinical standards support stable procedure volumes, while procurement is shaped by competitive tendering and strict quality documentation. Compliance with evolving regulatory requirements drives emphasis on clinical evidence, vigilance reporting, and supply-chain transparency. Country-level nuances matter: some markets favor motion preservation in cervical cases, while others emphasize cost-optimized fusion constructs. Hospitals prioritize system compatibility with imaging and navigation, and increasingly scrutinize tray rationalization to reduce sterilization burden and environmental impact.Asia-Pacific
Rapid capacity expansion and surgeon training broaden access to advanced spine care, with significant variability across health systems. Price sensitivity and public procurement drive interest in value-engineered implants that still meet performance standards. Local manufacturing and technology transfer gain importance, especially where authorities promote domestic innovation. Mature markets emphasize robotics and navigation for complex deformity and revision, while emerging markets focus on reliable, versatile systems suited to high-throughput degenerative care. Patient education and postoperative rehabilitation infrastructure influence real-world outcomes.Middle East & Africa
Specialty centers in the Gulf invest in cutting-edge platforms and attract regional medical travel for complex cases. Public and private payers encourage protocols that shorten hospital stays and standardize outcomes. In broader MEA, access remains uneven, with growth tied to surgical training, equipment financing, and maintenance support. Distributors with robust clinical education and after-sales service play an outsized role. Priorities include infection control, dependable supply, and implants that balance performance with logistical simplicity in resource-constrained settings.South & Central America
Procedure volumes are shaped by public-sector budgets and private insurance penetration, creating a two-speed market. Hospitals value systems that reduce OR time, minimize inventory complexity, and demonstrate consistent fusion outcomes. Regulatory pathways emphasize quality and post-market monitoring, while currency fluctuations and import dynamics affect pricing and tender timing. Training partnerships and local service capabilities differentiate vendors. Growing adoption of MIS techniques proceeds alongside ongoing demand for proven posterior fixation in degenerative and trauma indications.Spinal Implants Market Segmentation
By Thoracic Fusion and Lumbar Fusion Devices
- Posterior
- Interbody
- Anterior
By Cervical Fusion Devices
- Anterior
- Posterior
By Vertebral Compression Fracture Treatment Devices
- Balloon Kyphoplasty Devices
- Vertebroplasty Devices
By Spine Biologics
- Demineralized Bone Matrix
- Bone Morphogenetic Proteins
- Bone Substitutes
- Machined Bones
- Cell-Based Matrices
- Allograft Bone
By Spinal Decompression Devices
- Discectomy
- (Laminoplasty
- Laminectomy
- and Laminotomy)
- Foraminotomy and Foraminectomy
- Facetectomy
- Corpectomy
Key Market players
Medtronic, DePuy Synthes, Stryker, Zimmer Biomet, Globus Medical, NuVasive, B. Braun Aesculap, Orthofix, Alphatec Spine (ATEC), Medacta, Spineart, Xtant Medical, Spinal Elements, Centinel Spine, Ulrich MedicalSpinal Implants Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Spinal Implants Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Spinal Implants market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Spinal Implants market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Spinal Implants market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Spinal Implants market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Spinal Implants market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Spinal Implants value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Spinal Implants industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Spinal Implants Market Report
- Global Spinal Implants market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Spinal Implants trade, costs, and supply chains
- Spinal Implants market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Spinal Implants market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Spinal Implants market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Spinal Implants supply chain analysis
- Spinal Implants trade analysis, Spinal Implants market price analysis, and Spinal Implants supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Spinal Implants market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medtronic
- DePuy Synthes
- Stryker
- Zimmer Biomet
- Globus Medical
- NuVasive
- B. Braun Aesculap
- Orthofix
- Alphatec Spine (ATEC)
- Medacta
- Spineart
- Xtant Medical
- Spinal Elements
- Centinel Spine
- Ulrich Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
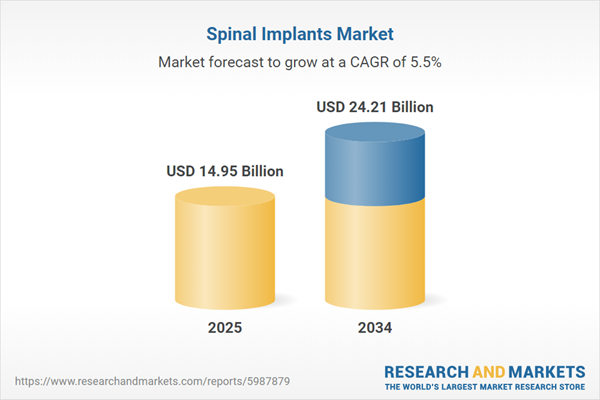
| Estimated Market Value ( USD | $ 14.95 Billion |
| Forecasted Market Value ( USD | $ 24.21 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |