Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these strong growth factors, the market encounters a significant obstacle regarding the high implementation expenses of advanced molecular diagnostic platforms. This financial hurdle restricts the adoption of modern testing solutions in resource-limited settings, which are often the regions where shigellosis is most endemic. Furthermore, the lack of adequate laboratory infrastructure in these developing areas complicates the application of comprehensive testing protocols, thereby hindering the market's global reach and expansion.
Market Drivers
The escalating burden of multidrug-resistant Shigella strains acts as a primary catalyst for the Global Shigella Test Kit Market. As resistance to standard first-line antibiotics like azithromycin and ciprofloxacin intensifies, clinical laboratories are urged to implement advanced diagnostic solutions capable of identifying both the pathogen and specific resistance markers to ensure effective therapy.This urgency is emphasized by recent surveillance data on the spread of dangerous variants; the California Department of Public Health reported in August 2024 that the percentage of Shigella isolates identified as extensively drug-resistant (XDR) in California increased to 12% between January and May 2024, a significant rise from 6.8% the previous year. Consequently, healthcare providers are prioritizing test kits that support accurate clinical decision-making to manage these complex, hard-to-treat infections.
Technological progress in molecular diagnostics further drives market growth by facilitating rapid, high-throughput detection via syndromic testing panels. The transition from traditional culture methods to multiplex polymerase chain reaction (PCR) systems enables the simultaneous identification of Shigella and other enteric pathogens, significantly reducing turnaround times and enhancing outbreak response. This demand for rapid diagnostics is evident in recent industry performance; BioMérieux reported in March 2024 that sales for its BIOFIRE non-respiratory panels, which include gastrointestinal testing, grew by 20% in the fourth quarter of 2023. These advanced tools are vital for managing the substantial disease burden, as the Centers for Disease Control and Prevention estimated in 2024 that approximately 450,000 Shigella infections occur annually in the United States alone.
Market Challenges
The substantial implementation costs associated with advanced molecular diagnostic platforms present a major impediment to the growth of the Global Shigella Test Kit Market. This financial barrier severely limits the adoption of modern testing solutions in resource-limited economies, which represent the regions with the highest endemic burden of the disease. Since healthcare budgets in these developing areas are often restricted, facilities are unable to procure or maintain the expensive instrumentation required for precise sensitivity profiling. Consequently, the market faces a structural ceiling on its expansion, remaining unable to capitalize on the demand in geographies where the clinical need is most acute.The insufficiency of laboratory infrastructure in these regions further complicates the deployment of necessary testing protocols. This inability to establish robust diagnostic frameworks prevents the identification of complex resistant strains, thereby limiting the market's reach. The severity of this issue is underscored by recent resistance trends that require such advanced detection; according to the California Department of Public Health, in 2024, the prevalence of extensively drug-resistant Shigella isolates rose to 12 percent between January and May, reflecting a 78 percent increase compared to the previous year. This escalation in resistance highlights the critical need for advanced diagnostics, yet the prohibitive costs associated with these tools continue to hamper their market penetration and overall industry growth.
Market Trends
The widespread adoption of multiplex syndromic gastrointestinal panels is transforming the market by addressing the clinical difficulty of distinguishing shigellosis from infections caused by other enteric pathogens with identical symptom profiles. Unlike traditional single-target tests, these comprehensive panels allow for the simultaneous detection of a wide array of bacteria, viruses, and parasites, thereby reducing diagnostic uncertainty and optimizing patient management in complex clinical scenarios. This trend toward broader diagnostic granularity is illustrated by recent product launches; for instance, Qiagen announced in June 2024 the U.S. launch of its QIAstat-Dx Gastrointestinal Panel 2, which enables the rapid identification of up to 16 common gastrointestinal pathogens in approximately one hour.Simultaneously, the integration of automated real-time PCR assays in clinical laboratories is accelerating as facilities seek to streamline high-volume diagnostic workflows and alleviate chronic staff shortages. Healthcare providers are increasingly adopting fully automated molecular platforms that minimize manual handling and enhance throughput, allowing for the efficient processing of routine gastrointestinal samples beyond the capacity of manual methods. This operational shift toward automation is driving significant commercial uptake; Seegene Inc. reported in May 2024 that sales for its gastrointestinal diagnostic products grew by 36 percent year-over-year, a performance attributed to the successful acquisition of new customers transitioning to advanced automated solutions.
Key Players Profiled in the Shigella Test Kit Market
- Thermo Fisher Scientific Inc.
- Meridian Bioscience, Inc.
- QIAGEN N.V.
- Diasorin S.p.A.
- Bio-Rad Laboratories Inc.
- BioMerieux SA
- Hygiena LLC
- Molbio Diagnostics Pvt. Ltd.
- Fujirebio Diagnostics, Inc.
- CTK Biotech, Inc.
Report Scope
In this report, the Global Shigella Test Kit Market has been segmented into the following categories:Shigella Test Kit Market, by Test Type:
- Lateral flow assays
- Enzyme-linked immunosorbent assay (ELISA)
- Polymerase chain reaction (PCR) assay
- Others
Shigella Test Kit Market, by Technology:
- Conventional diagnostic tests
- Nucleic acid-based tests
- Others
Shigella Test Kit Market, by End User:
- Hospitals
- Diagnostic laboratories
- Research institutes
- Others
Shigella Test Kit Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Shigella Test Kit Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Shigella Test Kit market report include:- Thermo Fisher Scientific Inc.
- Meridian Bioscience, Inc.
- QIAGEN N.V.
- Diasorin S.p.A.
- Bio-Rad Laboratories Inc.
- BioMerieux SA
- Hygiena LLC
- Molbio Diagnostics Pvt. Ltd.
- Fujirebio Diagnostics, Inc.
- CTK Biotech, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
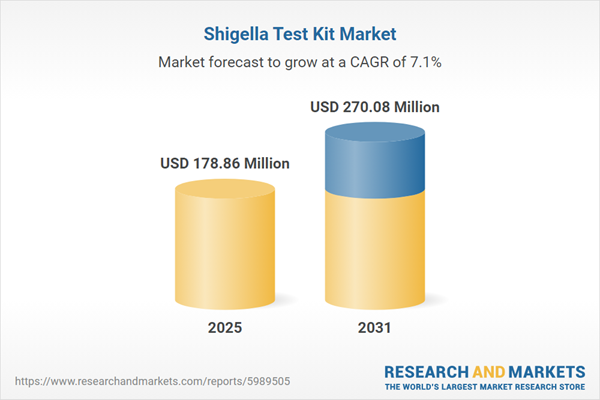
| Estimated Market Value ( USD | $ 178.86 Million |
| Forecasted Market Value ( USD | $ 270.08 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |