These drivers underscore the critical role of ATP assays in healthcare, research, and industrial sectors, facilitating advancements in personalized medicine, drug discovery, environmental monitoring, food safety and quality testing, and disease management. The 2022 WHO report highlights that food contamination affects about 600 million people globally, leading to over 200 diseases annually, including severe conditions such as diarrhea, resulting in 420,000 deaths and 33 million healthy life years lost.
The increasing prevalence of chronic diseases, such as cancer, diabetes, cardiovascular disorders, and infectious diseases, drives the demand for ATP assays in diagnostic testing and disease management. According to the report published by World Health Organization (WHO) in July 2.02 trillion people live with hepatitis B and 10 million with hepatitis C, leading to cancer, liver cirrhosis, and viral hepatitis-related deaths, and around 3 million new infections occur annually, with most going undetected in the Western Pacific Region. ATP levels are indicative of cellular dysfunction and disease progression, making these assays valuable tools for disease diagnosis, prognosis, and monitoring therapeutic responses. As healthcare systems worldwide face the challenge of managing chronic conditions, assays contribute to improving patient outcomes through early detection and personalized treatment strategies.
Contaminated food contains harmful pathogens such as bacteria, viruses, parasites, and chemical substances, posing significant health risks. ATP assays are pivotal in combating food contamination by swiftly and sensitively detecting microbial presence. These assays measure adenosine triphosphate, a universal indicator of microbial activity found in all living cells. ATP levels serve as reliable markers for assessing cleanliness in food surfaces, equipment, and processing areas. They enable prompt identification of contamination sources, empowering food manufacturers, processors, and regulators to take immediate corrective actions to prevent foodborne illnesses. In food processing, these products monitor hygiene practices, validate cleaning procedures, ensure compliance with safety standards, and enable real-time microbial monitoring to uphold food quality and safety across supply chains.
Technological innovations play a pivotal role in driving the ATP assays industry forward. Advances in assay sensitivity, accuracy, and automation have enhanced the performance and reliability of AP detection methods. Manufacturers are developing novel platforms that integrate cutting-edge technologies such as bioluminescence, fluorescence, and chemiluminescence to provide rapid and precise measurements. For instance, The RealTime-Glo Extracellular ATP Assay by Promega Corporation is a bioluminescent method designed to detect ATP released from cells undergoing stress, activation, or apoptosis. It serves as a pivotal biomarker for assessing treatments that may induce immunogenic cell death, a significant aspect of immune response modulation. These advancements cater to the growing demand for high-throughput screening and automated workflows in research laboratories and diagnostic settings.
The ATP assays industry is witnessing notable product innovation and expansion, with recent developments aimed at enhancing assay sensitivity, convenience, and affordability. A key example is Biotium’s launch of the Steady-ATP HTS Viability Assay Kit in March 2025, which reflects the industry’s focus on supporting high-throughput screening (HTS) applications in pharmaceutical and biotech research. The newly launched assay offers ultra-high sensitivity, detecting as few as 16 cells per well in a 384-well format, and delivers a stable luminescent signal with an extended half-life of over 5 hours. Its single-step, homogeneous workflow is designed to simplify assay protocols, making it highly compatible with automation and HTS systems. Importantly, the assay delivers performance comparable to premium market alternatives, such as CellTiter-Glo, while being positioned at a more accessible price point. This development is expected to drive broader adoption of luminescent ATP assays, particularly in budget-sensitive research laboratories and emerging biotech companies, supporting the overall growth trajectory of the ATP assays industry. Furthermore, the focus on user-friendly and cost-effective assays aligns with the market’s shift toward enabling real-time, scalable, and high-efficiency cell viability and metabolic studies across various research and quality testing domains.
Global ATP Assays Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For this report, the analyst has segmented the global ATP assays market report based on type, application, end use, and region:Type Outlook (Revenue, USD Million, 2018-2030)
- Luminometric ATP Assays
- Enzymatic ATP Assays
- Bioluminescence Resonance Energy Transfer (BRET) ATP Assays
- Cell-based ATP Assays
- Others
Application Outlook (Revenue, USD Million, 2018-2030)
- Drug Discovery & Development
- Clinical Diagnostics
- Environmental Testing
- Food Safety & Quality Testing
- Others
End Use Outlook (Revenue, USD Million, 2018-2030)
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Hospital & Diagnostics Laboratories
Regional Outlook (Revenue, USD Million, 2018-2030)
- North America
- U.S.
- Canada
- Mexico
- Europe
- UK
- Norway
- Sweden
- Denmark
- Spain
- Italy
- France
- Germany
- Asia-Pacific
- Japan
- China
- India
- South Korea
- Australia
- Thailand
- Latin America
- Brazil
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
Why Should You Buy This Report?
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The major companies featured in this ATP Assays market report include:- Thermo Fisher Scientific
- Promega Corporation
- PerkinElmer Inc.
- Becton, Dickinson and Company (BD)
- Lonza Group Ltd.
- Danaher Corporation
- Abcam plc
- Quest Diagnostics Incorporated
- Biomerieux SA
- 3M Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
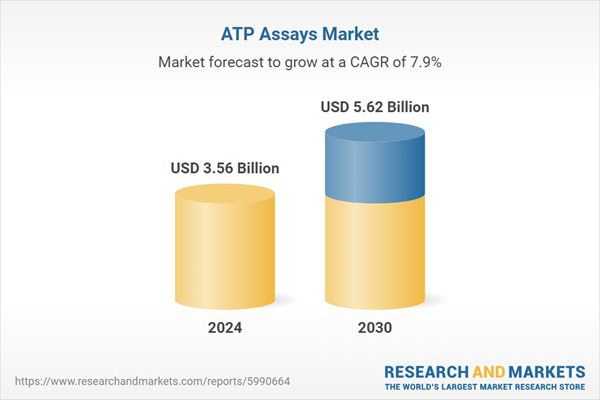
| Estimated Market Value ( USD | $ 3.56 Billion |
| Forecasted Market Value ( USD | $ 5.62 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |