Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, a substantial obstacle hindering broader market expansion is the high cost linked to advanced molecular diagnostic technologies, which limits their adoption in developing regions with insufficient healthcare infrastructure. This economic disparity results in a gap in effective disease management, restricting the market's reach in low-resource settings where it is frequently most needed. According to the American Cancer Society, an estimated 13,360 new cases of invasive cervical cancer will be diagnosed in the United States in 2025, emphasizing the enduring need for accessible and dependable diagnostic interventions to prevent disease progression.
Market Drivers
The transition from traditional cytology to advanced molecular diagnostics, specifically HPV DNA testing and self-collection techniques, acts as a primary market accelerator. These technological advancements improve screening adherence by providing less invasive options, thus extending diagnostic access to under-screened populations. For instance, Becton, Dickinson and Company announced in a May 2024 press release that the US Food and Drug Administration approved their HPV assay for use with self-collected vaginal specimens in a healthcare setting. Such regulatory achievements encourage the adoption of high-precision tools over conventional Pap smears, compelling laboratories to upgrade their diagnostic infrastructure to maintain clinical efficacy.Simultaneously, the rigorous enforcement of government-funded screening programs and international financial pledges significantly drives market expansion. Global initiatives focus on reducing disease burden through major investments that subsidize screening costs and improve infrastructure in low-to-middle-income regions. According to a March 2024 World Health Organization news release, governments and donors pledged nearly $600 million to expand global access to vaccination, screening, and treatment tools. This funding directly supports the procurement of diagnostic kits and colposcopy devices needed to meet elimination targets, which is essential given that the World Health Organization identified cervical cancer as the fourth most common cancer in women globally in 2024.
Market Challenges
The high costs associated with advanced molecular diagnostic technologies present a significant restraint on the growth of the global cervical dysplasia diagnostic market. In developing regions with limited healthcare infrastructure, the financial burden of acquiring and maintaining automated screening platforms frequently exceeds available budgets. This economic pressure forces healthcare facilities to rely on less expensive methods or to significantly restrict screening coverage. Consequently, manufacturers struggle to establish a presence in these low-resource areas, which limits the overall volume of test utilization.This pricing barrier creates a severe imbalance in market penetration, leaving high-risk populations underserved despite the clear need for early detection interventions. Data from the World Health Organization in 2024 indicates that approximately 94% of all cervical cancer deaths worldwide occurred in low- and middle-income countries. This figure demonstrates that regions with the highest clinical burden are currently unable to access modern diagnostic solutions due to economic constraints. Therefore, the inability to align product pricing with the purchasing power of these developing economies restricts the total addressable market.
Market Trends
The integration of Artificial Intelligence into digital pathology and cytology is fundamentally transforming laboratory workflows by improving diagnostic precision and operational efficiency. Diagnostic centers are increasingly utilizing deep-learning algorithms to analyze digitized slide images, identifying precancerous lesions with higher sensitivity than manual microscopic reviews. This technological shift automates the prioritization of high-risk samples, directly addressing human error risks in high-volume screening environments. For example, Hologic Inc. reported in a February 2024 press release that their FDA-cleared Genius Digital Diagnostics System achieved a 28% reduction in false-negatives for high-grade squamous intraepithelial lesions compared to traditional microscopic review, setting a new standard for screening accuracy.Concurrently, the adoption of p16/Ki-67 dual-staining biomarkers for triage is emerging as a critical trend for managing patients with HPV-positive results. This immunocytochemical method detects the simultaneous presence of two specific proteins to identify transforming infections, offering superior specificity over conventional Pap cytology for risk stratification. By accurately distinguishing between transient infections and those progressing to malignancy, this approach significantly optimizes patient management and reduces unnecessary follow-up procedures. According to a March 2024 Roche Diagnostics press release regarding new clinical guidelines, data from the IMPACT trial showed that this dual-stain technology detected cervical disease earlier than Pap cytology in seven out of ten HPV-positive women, validating its growing role in clinical practice.
Key Players Profiled in the Cervical Dysplasia Diagnostic Market
- Abbott Laboratories Inc.
- Becton Dickinson & Company
- F. Hoffmann-La Roche Ltd.
- Hologic Inc.
- QIAGEN, LLC
- Quest Diagnostics Incorporated
- CooperSurgical, Inc.
- Micromedic Technologies Ltd.
- Karl Kaps GmbH & Co. KG
Report Scope
In this report, the Global Cervical Dysplasia Diagnostic Market has been segmented into the following categories:Cervical Dysplasia Diagnostic Market, by Product Type:
- Diagnostic Tests
- Diagnostic Devices
- Others
Cervical Dysplasia Diagnostic Market, by End User:
- Hospitals
- Diagnostic Centers
- Research & Academic Institutes
- Ambulatory Surgical Centers
- Others
Cervical Dysplasia Diagnostic Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cervical Dysplasia Diagnostic Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cervical Dysplasia Diagnostic market report include:- Abbott Laboratories Inc.
- Becton Dickinson & Company
- F. Hoffmann-La Roche Ltd
- Hologic Inc.
- QIAGEN, LLC
- Quest Diagnostics Incorporated
- CooperSurgical, Inc.
- Micromedic Technologies Ltd.
- Karl Kaps GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
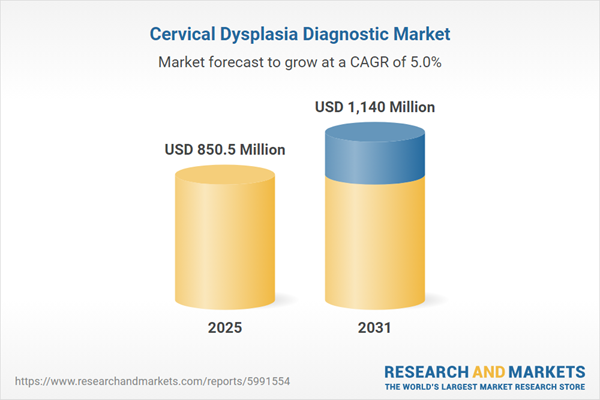
| Estimated Market Value ( USD | $ 850.5 Million |
| Forecasted Market Value ( USD | $ 1140 Million |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |