Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The Europe Genomic Cancer Panel and Profiling Market is a rapidly evolving sector, driven by advances in genomic technologies and the increasing incidence of cancer across the continent. This market encompasses various diagnostic tools and services designed to analyze genetic mutations and variations associated with different cancer types. These genomic cancer panels and profiling technologies enable personalized medicine approaches, allowing for tailored treatment strategies based on an individual's genetic makeup. The market growth is fueled by the rising demand for precision oncology, the increasing adoption of next-generation sequencing (NGS) technologies, and the growing awareness of the benefits of early cancer detection and targeted therapies. Key players in the market are investing heavily in research and development to enhance the accuracy and efficiency of genomic testing. Collaborations between biotech companies, academic institutions, and healthcare providers are also accelerating the development of innovative genomic solutions. Government initiatives and funding aimed at advancing cancer research and improving healthcare infrastructure are significant contributors to market expansion.
The market is segmented based on product type, cancer type, end-user, and geography. Product types include comprehensive genomic profiling tests, gene panels, and other sequencing technologies. Common cancer types profiled include breast cancer, lung cancer, colorectal cancer, and others. End-users range from hospitals and diagnostic laboratories to research institutes and academic centers. Challenges in the market include high costs of genomic testing, ethical concerns related to genetic data privacy, and the need for standardization of testing protocols. However, ongoing advancements in technology and the growing trend of integrating artificial intelligence and machine learning in genomic data analysis are expected to mitigate these challenges.
Key Market Drivers
The increasing incidence of cancer
The increasing incidence of cancer across Europe presents a significant healthcare challenge, with profound implications for both patients and healthcare systems. Cancer is now one of the leading causes of mortality in Europe, accounting for a substantial burden of disease and placing immense pressure on healthcare resources. As the population ages and lifestyles evolve, the prevalence of cancer continues to rise, underscoring the urgent need for advanced diagnostic and therapeutic solutions.Genomic cancer profiling emerges as a crucial tool in addressing this pressing healthcare need. By analyzing the genetic makeup of tumors, genomic profiling enables early detection of cancer, often at a stage when treatment options are most effective. It facilitates precise diagnosis by identifying specific genetic mutations and alterations associated with different cancer types. This molecular characterization not only helps oncologists tailor treatment strategies but also predicts the likelihood of treatment response and potential side effects, enhancing overall patient care.
Genomic profiling plays a pivotal role in personalized medicine, a paradigm shift in oncology that emphasizes individualized treatment approaches based on a patient's unique genetic profile. By identifying molecular targets and biomarkers, genomic testing enables the selection of targeted therapies that are more likely to be effective, sparing patients from unnecessary treatments and minimizing adverse effects. This personalized approach not only improves treatment outcomes but also enhances the patient’s experience, offering hope and optimism amidst the challenges of cancer diagnosis and treatment.
The increasing incidence of cancer in Europe underscores the critical importance of genomic cancer profiling as a frontline tool in the fight against cancer. By enabling early detection, accurate diagnosis, and personalized treatment, genomic profiling has the potential to transform cancer care, improving outcomes and quality of life for patients across the continent. As research advances and technology evolves, genomic cancer profiling will continue to play an indispensable role in shaping the future of oncology, offering hope for a world where cancer is no longer a leading cause of mortality.
Technological Advancements
Next-generation sequencing (NGS) technologies represent a significant leap forward in genomic analysis, revolutionizing the landscape of cancer profiling. These cutting-edge sequencing platforms have transformed the way genetic information is decoded, offering unprecedented speed, accuracy, and cost-effectiveness compared to traditional sequencing methods. The integration of NGS in cancer profiling has opened new frontiers in our understanding of the genetic basis of cancer and has become a cornerstone of precision oncology. One of the primary advantages of NGS technology is its ability to simultaneously analyze multiple genes in a single sequencing run. This multiplexing capability allows for comprehensive profiling of cancer genomes, enabling the detection of a wide range of genetic alterations, including point mutations, insertions, deletions, and copy number variations. By comprehensively interrogating the entire cancer genome, NGS facilitates the identification of driver mutations and oncogenic pathways underlying various cancer types, paving the way for personalized treatment strategies tailored to each patient's unique genetic profile.The rapid advancements in NGS technology have fueled its widespread adoption in clinical and research settings alike. Continuous improvements in sequencing platforms, bioinformatics tools, and data analysis algorithms have further enhanced the performance and reliability of NGS-based cancer profiling assays. As a result, NGS has become an indispensable tool for oncologists, enabling them to make more informed clinical decisions and optimize treatment outcomes for cancer patients.
The cost-effectiveness of NGS has significantly lowered the barrier to entry for genomic cancer profiling, making it accessible to a broader range of healthcare providers and patients. The decreasing cost of sequencing and the scalability of NGS platforms have democratized genomic medicine, democratizing access to cutting-edge diagnostic technologies and personalized treatment options for cancer patients across Europe.
Key Market Challenges
High Costs of Genomic Testing
The high costs associated with genomic cancer profiling pose a significant challenge to widespread adoption and accessibility. These tests involve sophisticated laboratory techniques, advanced equipment, and specialized expertise, all of which contribute to their considerable expense. The complexity of genomic data analysis further drives up the cost of testing, as it requires specialized bioinformatics tools and computational resources. However, despite the initial high costs, ongoing technological advancements are expected to lead to cost reductions over time. As sequencing technologies become more efficient and streamlined, economies of scale are achieved, driving down the per-sample cost of genomic testing. Innovations in sample preparation, sequencing chemistry, and data analysis algorithms contribute to cost savings, making genomic profiling more affordable and accessible to a broader population.The increasing coverage of genomic testing by health insurance companies and government healthcare programs helps mitigate the financial burden on patients. Many insurers now recognize the clinical utility of genomic testing and provide coverage for specific indications, such as guiding targeted cancer therapies or identifying hereditary cancer risk. Government-funded initiatives and research grants support the development and adoption of genomic testing, further expanding access to these innovative diagnostic tools.
Ethical and Data Privacy Concerns
Ethical and data privacy concerns represent significant challenges in the genomic cancer profiling market, raising important questions about patient consent, data ownership, and confidentiality. Genomic testing generates vast amounts of highly sensitive genetic data, which can reveal predispositions to certain diseases, hereditary traits, and other personal information. Ensuring the privacy and security of this genetic information is paramount to maintaining patient trust and safeguarding individual rights.One of the primary ethical concerns in genomic cancer profiling is the potential for unintended consequences, such as genetic discrimination or stigmatization based on genetic predispositions to certain diseases. Patients may fear that genetic testing results could be used against them by insurers, employers, or other entities, leading to discrimination or adverse treatment. There are concerns about the implications of genetic testing for family members, as genetic information can have implications beyond the individual being tested.
To address these concerns, regulatory frameworks and data protection measures are being implemented to ensure the responsible use and management of genetic data. Legal and ethical guidelines govern the collection, storage, and sharing of genetic information, requiring informed consent from patients and stringent data security protocols to protect against unauthorized access or misuse. Initiatives such as the General Data Protection Regulation (GDPR) in Europe provide robust data privacy protections and rights for individuals, including the right to access, rectify, and delete personal data.
Key Market Trends
Integration of Artificial Intelligence and Machine Learning
The integration of artificial intelligence (AI) and machine learning (ML) into genomic data analysis represents a transformative leap forward in cancer profiling, offering unparalleled accuracy, efficiency, and insight into the complex genetic landscape of cancer. AI and ML algorithms have the unique ability to process massive volumes of genomic data, identify intricate patterns, and predict outcomes with a level of precision and speed that surpasses human capabilities. This technological synergy not only enhances the capabilities of genomic cancer panels but also accelerates their adoption across clinical and research settings.One of the key advantages of AI and ML in genomic data analysis is their ability to uncover hidden patterns and correlations within large datasets that may elude traditional analytical methods. By leveraging sophisticated algorithms, AI and ML can identify subtle genetic variations, biomarkers, and molecular signatures associated with specific cancer subtypes, enabling more precise diagnosis and treatment selection. This advanced data mining capability enhances the sensitivity and specificity of genomic cancer panels, improving their clinical utility and relevance in oncology practice.
AI and ML algorithms excel at predictive modeling, enabling them to forecast disease progression, treatment response, and patient outcomes based on genomic data. By integrating multi-omics data sources, such as genomics, transcriptomics, proteomics, and metabolomics, AI-driven models can generate comprehensive molecular profiles of individual tumors, guiding personalized treatment strategies tailored to each patient's unique genetic makeup. This predictive modeling capability empowers oncologists to make more informed clinical decisions, optimize treatment regimens, and improve patient outcomes.
The iterative nature of AI and ML algorithms enables continuous learning and refinement over time, as they analyze increasingly diverse and complex datasets. This iterative learning process enhances the robustness and adaptability of AI-driven models, ensuring their relevance and efficacy in the dynamic field of oncology. As AI and ML algorithms evolve, they hold the potential to revolutionize cancer care by enabling truly personalized and data-driven approaches to diagnosis, treatment, and precision medicine.
Government Initiatives and Funding
Government initiatives and funding play a pivotal role in driving advancements in cancer research and fostering innovation in genomic cancer profiling across Europe. With cancer being a significant public health concern, governments recognize the importance of investing in research infrastructure, fostering collaborations, and providing financial support to accelerate progress in cancer diagnostics and treatment. Government support for cancer research encompasses a range of initiatives, including the establishment of research centers, funding grants, and the allocation of resources to support scientific endeavors focused on understanding the molecular mechanisms underlying cancer development and progression. These initiatives aim to fuel discoveries in cancer biology, genetics, and genomics, laying the foundation for the development of novel diagnostic tools and therapeutic interventions.Government funding provides critical support for academic and research institutions engaged in cancer research, enabling scientists and clinicians to conduct groundbreaking studies and translate research findings into clinical applications. By investing in research infrastructure and providing grants, governments create an enabling environment for innovation and collaboration, fostering the development of cutting-edge genomic cancer panels and profiling technologies.Public-private partnerships are another key driver of innovation in the genomic cancer panel market. Collaborations between public institutions, such as universities and research centers, and private companies, including biotechnology firms and diagnostic laboratories, leverage complementary expertise and resources to accelerate the development and commercialization of genomic solutions.
These partnerships enable the translation of scientific discoveries into clinically relevant products and services, bridging the gap between research and clinical practice. By pooling together scientific knowledge, technical capabilities, and financial resources, public-private partnerships facilitate the rapid advancement and widespread adoption of genomic cancer panels, ultimately benefiting patients by providing access to state-of-the-art diagnostic tools and personalized treatment options. Public-private partnerships foster an ecosystem of innovation, driving continuous improvement and refinement of genomic cancer profiling technologies. By fostering collaboration and knowledge exchange between academia and industry, these partnerships stimulate innovation, spur technological advancements, and enhance the competitiveness of the genomic cancer panel market.
Segmental Insights
Application Insights
Based on the application, In 2023, the Europe Genomic Cancer Panel and Profiling Market witnessed the clinical application segment emerging as the dominant segment. There has been a growing emphasis on personalized medicine in oncology, with healthcare providers increasingly leveraging genomic profiling to tailor treatment strategies to individual patients. Genomic cancer panels provide detailed genetic information that guides the selection of targeted therapies, improving treatment outcomes and minimizing adverse effects. As a result, oncologists and clinicians are integrating genomic testing into routine clinical practice to optimize patient care.Advancements in sequencing technologies and bioinformatics tools have enhanced the accuracy, efficiency, and affordability of genomic testing, making it more accessible to healthcare providers and patients. Next-generation sequencing (NGS) technologies, in particular, have revolutionized genomic analysis, allowing for comprehensive profiling of cancer genomes and facilitating the identification of actionable genetic alterations. Regulatory approvals and reimbursement policies have played a significant role in driving the adoption of genomic cancer panels in clinical settings. Increasingly, health insurance companies and government healthcare programs are recognizing the clinical utility of genomic testing and providing coverage for specific indications, further incentivizing its use in clinical practice.
Country Insights
In 2023, United Kingdom emerged as dominated country in the Europe Genomic Cancer Panel and Profiling Market. UK benefits from a supportive regulatory framework and a resilient healthcare infrastructure, which have facilitated the seamless adoption of genomic testing in clinical settings. Regulatory bodies like the National Institute for Health and Care Excellence (NICE) have played a pivotal role in providing guidance on the utilization of genomic technologies in cancer care, instilling confidence among healthcare providers and driving further market expansion. The UK has made significant investments in genomic medicine through initiatives such as the Genomes Project and the Genomics England program. These initiatives have accelerated genomic research and data collection efforts, providing valuable insights into the genetic basis of cancer and paving the way for the implementation of genomic profiling in routine clinical care. The UK's commitment to genomic medicine has positioned it as a leader in the field and contributed to its dominant market share in the Europe Genomic Cancer Panel and Profiling Market.Recent Developments
- On January 3, 2023, Burning Rock, a company specializing in the application of next-generation sequencing (NGS) technology in precision oncology, announced that its OverC Multi-Cancer Detection Blood Test (MCDBT) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). Under the FDA's Breakthrough Devices Program, Breakthrough Device Designation is granted to select medical devices that offer more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases, such as cancer. This program aims to expedite the development, evaluation, and review of designated medical devices, providing patients and healthcare providers with expedited access to them.
Key Market Players
- Agilent Technologies Deutschland GmbH
- ARUP Laboratories
- Burning Rock Biotech Limited
- U.S. Caris MPI, Inc.
- Thermo Fisher Scientific Inc.
- Danaher Corporation
- Exact Sciences Europe, Ltd
- F. Hoffmann-La Roche Ltd
Report Scope:
In this report, the Europe Genomic Cancer Panel and Profiling Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Europe Genomic Cancer Panel and Profiling Market, By Application:
- Clinical
- Research
Europe Genomic Cancer Panel and Profiling Market, By End User:
- Hospitals
- Clinical and Diagnostic Laboratories
- Research and Academic Institutes
- Other End Users
Europe Genomic Cancer Panel and Profiling Market, By Country:
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Poland
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Europe Genomic Cancer Panel and Profiling Market.Available Customizations:
Europe Genomic Cancer Panel and Profiling Market report with the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Agilent Technologies Deutschland GmbH
- ARUP Laboratories
- Burning Rock Biotech Limited
- U.S. Caris MPI, Inc.
- Thermo Fisher Scientific Inc.
- Danaher Corporation
- Exact Sciences Europe, Ltd
- F. Hoffmann-La Roche Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 128 |
| Published | August 2024 |
| Forecast Period | 2023 - 2029 |
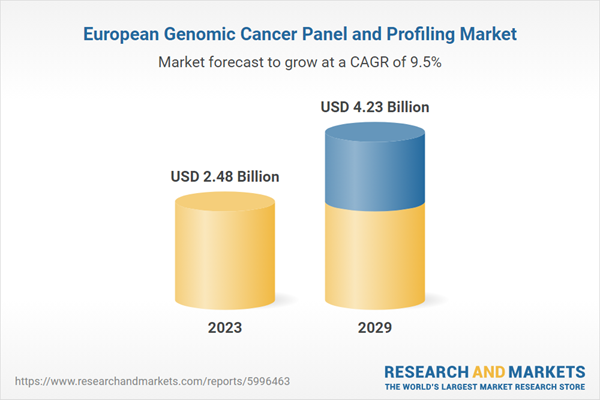
| Estimated Market Value ( USD | $ 2.48 Billion |
| Forecasted Market Value ( USD | $ 4.23 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 8 |