Speak directly to the analyst to clarify any post sales queries you may have.
An integrated strategic overview of cervical cancer therapeutics showcasing scientific progress, care delivery evolution, and stakeholder imperatives
The landscape of cervical cancer therapeutics has entered a period of sustained scientific momentum and shifting clinical priorities. Advances in targeted agents, immuno-oncology approaches, and vaccine science have expanded potential treatment paradigms, while evolving care delivery models and payer expectations are changing how therapies move from development into clinical practice. Stakeholders face converging pressures: the need to demonstrate meaningful patient benefit, to align with payer and health system value criteria, and to manage increasingly complex global supply chains. In this environment, clear strategic framing is essential to prioritize investments and to translate clinical innovation into accessible care pathways.Against this backdrop, it is important to recognize the heterogeneous therapy landscape that includes traditional cytotoxic chemotherapy, precision-targeted agents, immune-based approaches, and both prophylactic and therapeutic vaccine strategies. These modalities have distinct development pathways, regulatory considerations, and commercialization implications. Moreover, routes of administration, distribution channels, and end-user settings create additional layers of operational and commercial complexity. Consequently, decision-makers must integrate clinical evidence, health-economic perspectives, and pragmatic delivery considerations to build resilient programs that maximize patient access and clinical impact.
Emerging therapeutic paradigms and technological inflections reshaping cervical cancer treatment pathways clinical practice and research priorities
Clinical science and commercial practice are simultaneously converging to reshape how cervical cancer is prevented, detected, and treated. Immunotherapy innovations, including checkpoint inhibitors and adoptive cell approaches, are moving from experimental settings into broader clinical evaluation, while targeted strategies focused on kinase inhibition and DNA repair pathways are offering more precise options for molecularly defined patient groups. At the same time, vaccine science is bifurcating into stronger prophylactic prevention efforts and novel therapeutic vaccine concepts that seek to harness or educate the immune response against established disease. These therapeutic shifts are altering trial design, regulatory dialogues, and evidence-generation strategies.Concurrently, digital health, precision diagnostics, and decentralized care delivery are changing patient pathways. Rapid diagnostic platforms and biomarker-driven enrollment enable more efficient, adaptive clinical studies. Meanwhile, value-based contracting and outcome-based reimbursement models pressure sponsors to generate robust real-world evidence and to design patient-centric support programs. As a result, organizations that integrate translational science with pragmatic commercialization planning-aligning biomarkers, route of administration, distribution capabilities, and payer engagement-will be better positioned to accelerate adoption and to demonstrate sustainable value across healthcare systems.
How 2025 United States tariff measures are altering cervical cancer therapeutics supply chains procurement approaches and strategic cost management
The introduction of tariff measures emanating from the United States in 2025 has generated a new set of considerations for manufacturers, distributors, and health systems engaged in cervical cancer therapeutics. Supply chain economics and sourcing strategies are under renewed scrutiny as organizations assess the implications for imported active pharmaceutical ingredients, biologic components, cold-chain-dependent vaccine supplies, and finished dosage forms. In practical terms, procurement teams and contract manufacturers must re-evaluate supplier portfolios, contractual protections, and contingency inventories to mitigate exposure to cross-border cost adjustments and regulatory friction.Moreover, the tariff environment is catalyzing operational responses across the value chain. Some stakeholders are accelerating onshore or nearshore manufacturing partnerships to preserve cost stability and reduce lead-time variability. Others are renegotiating supply agreements or diversifying suppliers to preserve continuity of intravenous and intramuscular therapies and to protect cold-chain logistics for vaccines and biologics. In parallel, commercial teams are reassessing pricing strategies and payer dialogue to address potential downstream impacts on reimbursement and patient access. Ultimately, resilience will stem from integrated planning that links sourcing, manufacturing, logistics, and payer engagement to preserve therapeutic availability and care continuity.
Segmentation insights showing how therapy classes administration routes distribution end-user dynamics and therapy lines shape clinical and commercial strategy
Segmentation reveals critical decision points that shape clinical development, regulatory planning, and commercialization tactics. When therapies are examined by type, chemotherapy remains a mainstay with established regimens and supply models, where Platinum agents and Taxanes present familiar manufacturing and administration demands. Immunotherapy's emergence emphasizes both checkpoint inhibitors and adoptive cell therapy, each with unique clinical endpoints, biomarker needs, and operational requirements. Targeted therapy pathways prioritize kinase inhibitors and PARP inhibitors that demand molecular diagnostics and precision deployment, while vaccine strategies span prophylactic vaccines aimed at prevention and therapeutic vaccines intended to stimulate antitumor immunity.Route of administration materially influences clinical adoption and service delivery. Intramuscular and intravenous modalities require facility-based administration and logistics support, whereas oral and topical formulations enable broader decentralization and potential home-based management. Distribution channel dynamics shape access: hospital pharmacies maintain critical in-hospital supply and oncology infusion logistics, online pharmacies introduce new patient convenience models and adherence support opportunities, and retail pharmacies extend community access. End-user segmentation-clinics, home care, and hospitals-determines where care is delivered and influences reimbursement, training needs, and patient support. Finally, line-of-therapy considerations across first, second, and third-line settings inform trial design, comparative-effectiveness strategies, and value communication to payers and clinicians.
Regional intelligence describing access regulatory environment manufacturing and adoption dynamics that determine treatment uptake across major regions
Regional dynamics impose distinct regulatory, access, and operational contours that shape strategy. In the Americas, diverse payer mixes and strong clinical trial infrastructure often accelerate adoption for innovative agents, while concentrated public health programs can support large-scale prophylactic vaccination campaigns. In Europe, Middle East & Africa, regulatory harmonization efforts, variable reimbursement pathways, and heterogeneous healthcare capacities necessitate tailored access plans that account for country-level procurement processes and localized manufacturing partnerships. In Asia-Pacific, manufacturing scale and rapidly evolving reimbursement environments create both opportunity and complexity, with accelerated uptake in select markets balanced against access gaps in others.Given these differences, global strategies must incorporate region-specific evidence generation, localized pricing and contracting approaches, and targeted distribution frameworks. For example, cold-chain requirements for vaccines and certain biologics necessitate investment in regional logistics and training, while differing diagnostic capabilities across territories influence the feasibility of biomarker-driven programs. Transitioning clinical promise into meaningful patient outcomes therefore depends on pragmatic regionalization of commercial plans, alignment with public health initiatives, and partnerships that bridge local infrastructure limitations.
Company-level dynamics highlighting innovation focus partnership models pipeline and go-to-market tactics shaping cervical cancer therapeutic strategies
Company strategies within the cervical cancer therapeutic arena exhibit a balance of competition and collaboration that drives innovation and market shaping. Biopharmaceutical developers are increasingly pursuing combination regimens and biomarker-driven indications to differentiate clinical utility, while emerging biotech firms often focus on niche scientific mechanisms such as therapeutic vaccines or novel immune-modulatory constructs. Large-scale manufacturers and contract development and manufacturing organizations play a critical role in ensuring supply continuity, scaling production for complex biologics, and providing flexible capacity for rapid ramp-up when demand shifts.Strategic behaviors include licensing partnerships to accelerate late-stage development, co-commercialization agreements to broaden geographic reach, and alliances with diagnostic providers to enable companion testing. Commercially, companies are deploying value demonstration frameworks that blend randomized clinical trial evidence with real-world data to support payer discussions. Robust patient support programs, outcomes-based contracting pilots, and targeted medical education initiatives are increasingly central to ensuring uptake. Ultimately, organizations that align scientific differentiation with operational excellence and payer-centered evidence generation will secure competitive advantage and sustainable access.
Practical recommendations for industry to optimize R&D strengthen supply resilience engage payers and expand patient access across cervical cancer therapeutics
Industry leaders should prioritize a pragmatic set of actions that align clinical innovation with durable access. First, strengthen supply resilience by diversifying sourcing, investing in regional manufacturing partnerships, and establishing contractual safeguards that mitigate cross-border disruptions. This operational foundation enables consistent therapy availability and reduces exposure to external shocks. Second, accelerate biomarker-enabled development and companion diagnostics integration so that targeted therapies and combination regimens can be deployed with maximal clinical precision and payer alignment.Third, expand evidence strategies to include both randomized data and structured real-world evidence collection to support value-based discussions and outcome-based contracting. Fourth, tailor commercial pathways to administration route and end-user settings by developing home-based support for oral and topical options while preserving robust logistics and training for intramuscular and intravenous therapies. Finally, engage proactively with payers and public health stakeholders to co-design access programs that reflect population-level prevention priorities and individual patient needs. Taken together, these actions will translate scientific gains into equitable, sustainable care delivery.
Mixed-methods research approach integrating primary expert interviews targeted literature review triangulation and validation to ensure analytical rigor and trusted insights
This analysis is grounded in a mixed-methods research approach that integrates primary expert interviews with a targeted review of scientific literature, regulatory guidance, and clinical practice trends. Primary input was obtained through structured discussions with clinical investigators, supply-chain specialists, payer advisors, and commercialization leaders to capture diverse perspectives on therapeutic performance, operational constraints, and reimbursement dynamics. Secondary evidence review synthesized peer-reviewed publications, regulatory documents, clinical trial registries, and published clinical guidelines to corroborate expert insights and to map the evolving evidence base.Findings were triangulated through iterative validation exercises that compared qualitative insights with documented clinical outcomes and operational case studies. Limitations are acknowledged: differences in national reporting and evolving trial data can shift the evidence base over time, and stakeholder priorities may vary by organizational mission. Nevertheless, the methodological framework emphasizes reproducibility and transparency, and it prioritizes cross-validated conclusions that are actionable for clinical development and commercial planning.
Synthesis of strategic imperatives and advances providing pragmatic guidance for stakeholders navigating the evolving cervical cancer therapeutics landscape
In summary, the cervical cancer therapeutics landscape is characterized by convergent scientific innovation and complex delivery challenges. Progress in immunotherapy, targeted agents, and vaccine strategies offers meaningful clinical promise, yet translating that promise into accessible care requires deliberate alignment of R&D, manufacturing, payer engagement, and distribution planning. Segmentation by therapy type, route of administration, distribution channel, end-user setting, and line of therapy reveals concrete operational and commercial implications that must be addressed in tandem with evidence generation.Regional variation and policy shifts, including changes in tariff regimes and procurement dynamics, further underscore the need for resilient supply strategies and flexible commercial models. Companies that couple scientific differentiation with pragmatic execution-investing in local partnerships, robust evidence ecosystems, and patient-centric access programs-will be best positioned to deliver sustainable value for patients and health systems alike. The path forward requires coordinated, evidence-driven decisions that balance innovation with equitable access.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Cervical Cancer Therapeutics Market
Companies Mentioned
- AbbVie Inc.
- AstraZeneca PLC
- Biocon Limited
- Bristol-Myers Squibb Company
- Cipla Limited
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Fresenius SE & Co. KGaA
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Seagen Inc.
- Serum Institute of India Pvt. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
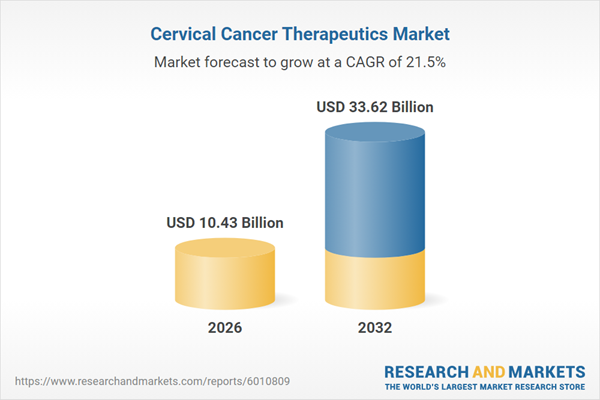
| Estimated Market Value ( USD | $ 10.43 Billion |
| Forecasted Market Value ( USD | $ 33.62 Billion |
| Compound Annual Growth Rate | 21.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |