Speak directly to the analyst to clarify any post sales queries you may have.
A concise overview of how clinical advances and strategic imperatives are reshaping treatment pathways and commercial approaches across chronic lymphocytic leukemia care
Chronic lymphocytic leukemia is transitioning from a disease historically managed with broad cytotoxic chemotherapy to one increasingly shaped by targeted and immune-directed therapies, creating both clinical opportunity and strategic complexity. In recent years, the cumulative clinical experience with agents that inhibit B-cell receptor signalling and with targeted apoptosis modulators has altered treatment paradigms, improved tolerability for many patients, and introduced new sequences of care that providers and payers must evaluate. Concurrently, the emergence of cellular therapies and investigational immunomodulatory approaches has broadened the therapeutic toolkit, prompting clinicians to reassess long-term management strategies and to consider durable remission as an attainable objective for selected patient populations.This executive summary frames the most consequential trends affecting product development, commercialization, and patient access. It synthesizes shifts in clinical practice, regulatory behavior, and supply chain dynamics that are already reshaping how stakeholders prioritize assets and invest in evidence generation. The content that follows aims to equip senior executives, clinical development leaders, and commercial strategists with actionable perspective on therapeutic differentiation, infrastructure needs, and partnership models that will determine success in the next phase of CLL care. The narrative emphasizes pragmatic implications, translating scientific advancement into operational requirements for clinical programs, market entry plans, and value demonstration efforts.
How molecularly targeted agents, advanced immunotherapies, regulatory evolution, and supply chain modernisation are jointly redefining clinical and commercial playbooks
Across chronic lymphocytic leukemia therapeutics, a series of transformative shifts is redefining clinical priorities and commercial models simultaneously. Targeted therapies that interrupt specific molecular drivers have moved from experimental to standard-of-care roles, prompting new sequence strategies and elevating the importance of biomarker stratification and resistance monitoring. At the same time, immunotherapy innovations, including engineered cellular products and next-generation monoclonal antibodies, are pushing durable response into view and necessitating new infrastructure for delivery and long-term follow-up.Beyond pharmacology, regulatory paradigms and evidence expectations are adapting to accelerated pathways and real-world data integration, altering the timelines and evidence packages required for reimbursement negotiations. Digital tools and decentralized trial designs are reducing patient burden and expanding trial access, while evolving payer frameworks emphasize value-based contracting and outcomes-based reimbursement. Manufacturing innovations and the rise of contract development and manufacturing organisations are enabling faster scale-up for complex biologics, but also increasing dependency on specialised suppliers. Collectively, these shifts are creating higher technical barriers to entry for late-stage differentiation while offering new commercialization vectors for organisations that can align clinical evidence with pragmatic delivery solutions and payer-centric value propositions.
Understanding how 2025 tariff shifts are prompting supply chain diversification, manufacturing adjustments, and clinical supply risk mitigation strategies across oncology therapeutics
The cumulative impact of recent tariff policy developments and trade measures in 2025 has introduced a new layer of complexity for pharmaceutical supply chains and procurement strategies relevant to chronic lymphocytic leukemia therapeutics. Sourcing of active pharmaceutical ingredients, specialty reagents for biologics manufacturing, and imported device components for cell therapy processing have all become subjects of heightened commercial scrutiny. As a result, manufacturers and procurement teams are recalibrating supplier portfolios, increasing hold inventories for critical inputs, and exploring alternative regional sources to mitigate single-country exposure.These adjustments have downstream implications for contract manufacturing timelines, costing assumptions, and the negotiation dynamics with payers who may push back on price increases that reflect changed import economics. Clinical trial sponsors face logistical challenges in ensuring uninterrupted investigational product supply to global sites, and trial budgets may require contingency provisions for customs delays and tariff-related cost shifts. In response, strategic options such as relocating certain manufacturing steps closer to major markets, qualifying multiple API suppliers, and leveraging tariff classification reviews have become common. Importantly, stakeholders that proactively integrate tariff risk into commercial modelling, supply chain contracts, and regulatory submissions will be better positioned to maintain continuity of care and to protect launch momentum in affected jurisdictions.
Detailed segmentation insights showing how therapeutic class, administration route, and distribution channel create distinct clinical, access, and commercial imperatives for stakeholders
A nuanced segmentation approach reveals meaningful differences in clinical positioning, development risk, and commercial execution for chronic lymphocytic leukemia therapies. Based on Therapeutic Class, market is studied across Chemotherapy, Combination Therapy, Immunotherapy, and Targeted Therapy. The Immunotherapy is further studied across CAR-T Therapy, Checkpoint Inhibitors, and Monoclonal Antibodies. The Targeted Therapy is further studied across BCL-2 Inhibitors, BTK Inhibitors, and PI3K Inhibitors. These therapeutic distinctions matter because they drive divergent clinical trial endpoints, safety monitoring needs, and infrastructure requirements; targeted and immunologic agents often demand biomarker programs and long-term follow-up, whereas traditional chemotherapeutic approaches emphasize dosing optimisation and supportive care pathways.Based on Mode Of Administration, market is studied across Intravenous and Oral. The route of administration influences adherence, site-of-care economics, and the design of patient support programs; oral therapies enable at-home dosing and digital adherence tools but raise adherence and drug interaction concerns, while intravenous options concentrate care in clinic settings and can strengthen provider involvement in long-term monitoring. Based on Distribution Channel, market is studied across Hospital Pharmacies, Retail Pharmacies, and Specialty Pharmacies. Each channel presents distinct access dynamics: hospital pharmacies support infusion and complex regimens, retail pharmacies offer broad geographic reach for community-dispensed oral drugs, and specialty pharmacies provide integrated patient support, prior authorization assistance, and adherence monitoring that are critical for complex or high-cost therapies.
How divergent regulatory frameworks, payer environments, and healthcare infrastructure across major regions are dictating tailored development and access strategies
Regional dynamics shape both how therapies are adopted clinically and how commercial strategies must be executed across major geographies. In the Americas, the United States remains a focal point for clinical development and early adoption due to its active investigator community, high density of specialised treatment centres, and established payer frameworks that facilitate innovative contracting models. The private and public payer mix in this region drives a need for robust value dossiers and payer engagement plans that demonstrate long-term outcomes and budget impact mitigation.Within Europe, Middle East & Africa, regulatory harmonisation under centralised procedures coexists with notable national-level variations in reimbursement and access, meaning that launch sequencing and local HEOR evidence generation are critical for rapid uptake. Countries with single-payer systems often require targeted economic models and registry data to support formulary placement. In Asia-Pacific, heterogeneity ranges from sophisticated regulatory environments with growing early-phase trial activity to markets where generics and biosimilar competition strongly influence pricing dynamics. In many Asia-Pacific markets, capacity constraints for complex cell therapies and variability in diagnostic access necessitate tailored deployment models that consider infrastructure investment and partnership with regional clinical centres. Cross-region collaborations, such as multinational trials and shared registries, are proving valuable in accelerating evidence generation and harmonising real-world outcome measures.
Competitive dynamics emphasise strategic alliances, manufacturing scale, and diagnostic integration as the primary levers for differentiation and accelerated adoption
The competitive landscape features a mix of incumbent pharmaceutical organisations with established hematology portfolios and nimble biotechnology companies pioneering next-generation modalities. Established players often leverage broad drug development experience, expansive distribution networks, and existing relationships with payer and provider communities to support product launches and lifecycle management. Conversely, smaller biotech firms frequently bring highly differentiated science, modular manufacturing approaches for cellular therapies, and focused clinical programs that target unmet biological mechanisms or specific patient subgroups.Strategic behaviours include licensing and co-development partnerships that pair clinical innovation with commercial scale, as well as alliances with contract manufacturers to de-risk production of complex biologics and cell therapies. Companies that invest early in biomarker assays and companion diagnostics create more defensible positioning for precision indications and smoother regulatory conversations. Moreover, entrants that prioritise integrated patient support, specialty pharmacy pathways, and digital adherence solutions tend to achieve stronger real-world persistence and patient satisfaction. Observed M&A activity and late-stage licensing deals indicate that pipeline synergies and access to manufacturing capacity are primary value drivers, with acquirers seeking to combine clinical differentiation with operational capabilities that reduce time-to-market hurdles.
Prioritised strategic actions for leadership teams to align clinical development, supply security, payer engagement, and patient support for durable commercial success
Industry leaders should prioritise a small set of high-impact actions to convert scientific progress into sustainable clinical and commercial success. First, invest in robust translational programs that link molecular profiling and resistance monitoring to clear clinical decision algorithms, thereby improving patient selection and strengthening payer discussions around value. Second, diversify supply chains for active ingredients and critical reagents while qualifying regional manufacturing options to mitigate tariff and logistics risks; this is particularly important for complex biologics and cell therapies. Third, design clinical programs that generate both regulatory-grade endpoints and real-world evidence, enabling flexible dossiers that can satisfy regulators, payers, and health systems simultaneously.Additionally, foster integrated launch models that coordinate hospital pharmacy, specialty pharmacy, and community dispensing to ensure seamless patient transitions between infusion care and oral maintenance. Engage early with payers to explore outcomes-based contracting and risk-sharing agreements that align reimbursement with longitudinal patient outcomes. Finally, build capabilities in digital patient support and remote monitoring to bolster adherence, capture real-world outcomes, and reduce the total cost of care. Collectively, these actions will help organisations reduce execution risk, accelerate uptake, and demonstrate measurable value to clinicians and payers alike.
Methodology blending regulatory analysis, clinical literature review, and expert interviews with iterative triangulation to validate clinical and commercial conclusions
The research underpinning this analysis combined structured secondary sourcing with primary expert engagement and iterative triangulation to ensure validity and relevance. Secondary inputs included peer-reviewed literature, clinical-trial registries, regulatory guidance documents, and published treatment guidelines to establish the scientific and evidentiary baseline. These sources were complemented by a programme of targeted primary interviews with hematologists, clinical trial investigators, payer advisers, and supply-chain leaders to capture contemporary operational constraints and frontline adoption behaviour.Quantitative and qualitative data streams were reconciled through triangulation, cross-checking clinical findings against regulatory filings and protocol designs, and validating commercial implications with payer and provider informants. The methodology also incorporated sensitivity reviews to identify areas where evidence is evolving rapidly, such as early-phase cellular therapy outcomes or emerging safety signals for novel targeted agents. Limitations include the natural lag between the latest trial disclosures and the cut-off for secondary literature, and variability in regional reporting standards that can influence access and uptake interpretations. Where appropriate, uncertainty is highlighted and recommendations are framed to accommodate iterative evidence updates.
Concluding perspective on how executional rigour, evidence excellence, and supply resilience will determine which innovations in chronic lymphocytic leukemia achieve sustained clinical and commercial impact
The chronic lymphocytic leukemia therapeutic environment is at an inflection point where scientific advances must be matched by strategic operational execution to realise patient and commercial value. The shift toward targeted therapies, refined immunologic approaches, and more patient-centric care delivery models offers meaningful improvements in tolerability and durable responses for many patients. However, these benefits come with increased complexity in manufacturing, distribution, evidence generation, and payer negotiation, requiring organisations to adopt multidisciplinary strategies that link clinical differentiation to pragmatic access plans.Ultimately, success will favour organisations that can rapidly translate mechanistic insights into clinically actionable programs, secure resilient supply and manufacturing pathways, and partner effectively with payers and providers to demonstrate real-world value. The recommendations provided here aim to help decision-makers prioritise investments, de-risk launches, and build the institutional capabilities necessary to compete in a landscape defined by precision medicine, complex biologics, and evolving reimbursement models. The next phase of progress in CLL will be determined as much by executional excellence as by scientific innovation.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
15. China Chronic Lymphocytic Leukemia Therapeutics Market
Companies Mentioned
The key companies profiled in this Chronic Lymphocytic Leukemia Therapeutics market report include:- AbbVie Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Bayer AG
- Bristol Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- Incyte Corporation
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Secura Bio, Inc.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
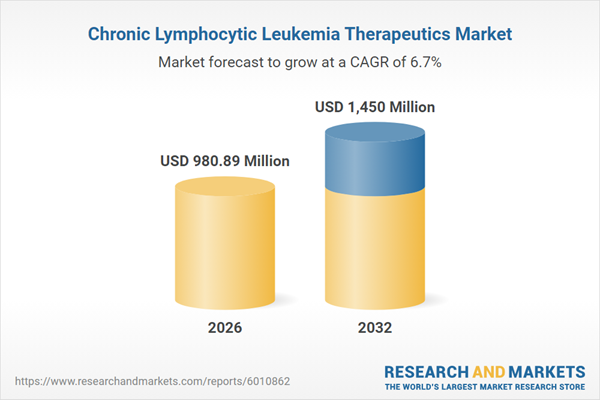
| Estimated Market Value ( USD | $ 980.89 Million |
| Forecasted Market Value ( USD | $ 1450 Million |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |