Speak directly to the analyst to clarify any post sales queries you may have.
A concise orientation to how slide stainer technologies shape laboratory workflows, operational reliability, and strategic procurement choices across clinical and research settings
Slide stainers sit at the intersection of laboratory throughput, diagnostic accuracy, and workflow efficiency, and this introduction frames the technology’s evolving role within histology and pathology laboratories. Historically positioned as specialized instruments for routine staining tasks, slide stainers now underpin faster turnaround times and reproducible staining quality, driving their adoption across diagnostic laboratories, hospitals, academic centers, and pharmaceutical research units. As laboratories pursue higher diagnostic certainty and greater capacity, the demand profile for equipment that balances reliability, ease of maintenance, and consumable compatibility has shifted markedly.Technological advances are reshaping expectations: automation and closed-consumable systems reduce operator variability, while reagent kit standardization and enhanced instrument software bring traceability and regulatory compliance into sharper focus. Simultaneously, procurement decisions are influenced by total lifecycle considerations, including service models, consumable availability, and integration with upstream and downstream digital pathology workflows. This introduction sets the stage for deeper analysis by documenting the converging forces-operational, technological, regulatory, and commercial-that are steering procurement, product development, and strategic investment across the slide stainer landscape.
How automation, digital pathology convergence, and shifting procurement models are reshaping product roadmaps and laboratory operations for slide stainer stakeholders
The landscape for slide stainers is experiencing transformative shifts driven by automation, digital integration, and evolving end-user expectations. Instrument vendors are accelerating the transition from legacy manual systems to fully automated platforms that deliver standardized staining protocols, improved batch tracking, and reduced hands-on technician time. This migration is catalyzing downstream changes in staffing models and quality-control practices, as laboratories shift from manual expertise toward instrument oversight and data management.Concurrently, digital pathology and image analysis are changing the value proposition for slide stainers; reproducible staining becomes a prerequisite for reliable algorithmic interpretation, prompting tighter reagent-instrument interoperability and increased emphasis on lot-to-lot consistency. Another critical shift is the emphasis on modular service and consumable ecosystems, where vendors bundle maintenance, reagent kits, and digital support into subscription-style offerings that smooth capital expenditure cycles. Lastly, macroeconomic and regulatory pressures are prompting organizations to prioritize supply chain resilience and regional sourcing strategies, further influencing product roadmaps and vendor selection criteria across the ecosystem.
Examining the cumulative operational and strategic effects of United States tariff actions in 2025 on sourcing, pricing dynamics, and supply chain resilience for slide stainer stakeholders
The cumulative effects of United States tariff changes implemented in 2025 have introduced tangible operational and commercial considerations for manufacturers, distributors, and end users of slide stainers and related consumables. Increased import duties on certain categories elevated landed costs for some equipment and reagent lines, prompting a reassessment of sourcing strategies and cost allocation across procurement, service contracts, and consumable pricing structures. These changes have not been uniform; they vary by product complexity, component origin, and the degree of vertical integration possessed by suppliers.In response, several manufacturers accelerated regional manufacturing or assembly to mitigate tariff exposure, while distributors restructured pricing models and extended lead times to protect margins. Laboratory buyers adjusted procurement timelines and explored multi-year service agreements to stabilize operational budgets. Equally, the tariff shift emphasized the importance of supply chain visibility and supplier diversification, urging stakeholders to develop contingency plans for reagent continuity and spare parts availability. The cumulative impact has been to increase emphasis on contractual flexibility, localized support capability, and the strategic alignment of purchasing policies with operational resilience and regulatory compliance.
A differentiated segmentation analysis revealing how category, product type, technology, automation level, and end-user profiles drive procurement priorities and lifecycle needs
A granular segmentation view clarifies product design choices, service requirements, and procurement preferences across different end-user profiles and laboratory footprints. Based on Category, equipment configurations are commonly differentiated between bench top units that prioritize compact footprints and floor standing systems that emphasize throughput and integrated automation; this distinction informs installation planning, maintenance access, and real estate utilization within laboratory layouts. Based on Product Type, the value chain spans consumables and accessories, equipment, and reagents and kits, each with distinct margin structures and service expectations; consumables and reagents tend to drive recurring revenue while equipment sales require robust aftermarket support and predictable spare parts availability.Based on Technology, staining approaches include Hematoxylin and Eosin for routine morphology, Immunohistochemistry for antigen-specific detection, and Special Staining, which itself is further studied across Cytochemical and Histochemical techniques; this technological segmentation shapes reagent complexity, validation needs, and training requirements. Based on Automation Level, laboratories choose among fully automated systems for high-throughput standardized workflows, semi-automated platforms for mixed-mode operations, and manual methods where flexibility or cost constraints dominate. Based on End User, demand manifests across academic and research institutes, diagnostic laboratories, hospitals, and pharmaceutical companies, with diagnostic laboratories further subdivided into hospital-based and independent facilities; each end-user segment carries different purchasing cycles, regulatory scrutiny, and service expectations that influence product roadmaps and customer engagement strategies.
Regional dynamics and operational priorities that determine adoption patterns, service models, and regulatory alignment for slide stainer deployments across global territories
Regional dynamics materially affect adoption patterns, regulatory frameworks, and supply chain design for slide stainers, producing differentiated strategic imperatives across major territories. In the Americas, buyer preference emphasizes rapid instrument deployment, integrated service networks, and compatibility with laboratory information systems; the region’s dense network of diagnostic laboratories and hospitals creates demand for high-throughput and reliable service coverage. Europe, Middle East & Africa presents a mixed landscape where regulatory heterogeneity and variable infrastructure levels prioritize modular system designs and flexible reagent offerings, while centralized public health procurement mechanisms in parts of the region influence vendor negotiations and tender responses. Asia-Pacific exhibits strong demand growth driven by expanding clinical testing capacity, substantial investment in private diagnostic networks, and a competitive local manufacturing ecosystem, all of which stimulate price sensitivity alongside a thirst for automation and workflow optimization.Cross-region comparisons underscore the importance of localized service models, regulatory alignment, and supply chain contingency planning. Vendors and purchasers alike must reconcile global product consistency with regional customization, balancing standardized reagent and staining protocols against local acceptance criteria and service expectations. This regional framing clarifies where investments in service hubs, training programs, and component localization will yield the greatest operational and commercial returns.
Strategic behaviors and competitive differentiators among slide stainer companies focusing on bundled solutions, aftermarket strength, and partnerships to broaden clinical utility
Competitive behavior among leading companies in the slide stainer ecosystem reveals strategic emphases on integrated solutions, aftermarket revenue, and partnerships that extend clinical utility. Companies are increasingly bundling instruments with proprietary reagent kits and comprehensive service contracts to capture recurring revenue and improve lifecycle predictability. Investment in automation and user-centric software interfaces has become a critical differentiator, as streamlined workflows reduce operator dependence and enable more consistent staining outcomes that integrate with digital pathology pipelines.Strategic collaborations and channel optimization are also prominent: suppliers partner with digital pathology and laboratory information system providers to offer end-to-end solutions, while distribution networks are refined to improve responsiveness and spare-part logistics. Some players prioritize local manufacturing and assembly to reduce exposure to cross-border trade disruptions and to meet regional regulatory requirements. Across the competitive landscape, the most effective companies balance technical innovation with robust service delivery, demonstrating that operational reliability and reagent consistency often trump headline performance metrics when laboratories evaluate total lifecycle value.
Actionable strategic priorities for industry leaders to strengthen automation, consumable ecosystems, supply resilience, and service models that drive lasting competitive advantage
Industry leaders should prioritize investments that enhance reproducibility, reduce total cost of ownership, and strengthen supply resilience to sustain competitive advantage. First, accelerating automation and software-enabled protocol standardization will deliver measurable labor efficiencies and higher-quality outputs, enabling institutions to reallocate skilled technicians toward value-add diagnostic tasks. Second, expanding consumable and reagent ecosystems under clear validation frameworks allows vendors to secure recurring revenue while offering end users simplified procurement and traceability, reducing administrative burden and variability in staining results.Third, diversifying manufacturing and distribution footprints-whether through regional assembly hubs, strategic supplier partnerships, or flexible contract manufacturing-will mitigate tariff exposure and logistics volatility. Fourth, bolstering service models with outcome-based contracts and digital remote diagnostics can reduce downtime and improve client satisfaction, making support an integral component of the value proposition. Finally, fostering interoperability with digital pathology platforms and laboratory information systems will ensure instruments remain central to modern diagnostic workflows, creating stickier customer relationships and enabling data-driven continuous improvement initiatives.
A transparent mixed-methods research approach blending stakeholder interviews, technical validation, and scenario analysis to ensure actionable and verifiable findings
The research approach combined structured qualitative inquiry with rigorous data triangulation to ensure findings reflect operational realities and stakeholder perspectives. Primary research included in-depth interviews with laboratory directors, histotechnologists, procurement professionals, and senior R&D leaders, capturing firsthand accounts of procurement criteria, service expectations, and the practical implications of automation adoption. These interviews were synthesized with vendor briefings and technical documentation to understand product roadmaps, validation practices, and consumable lifecycle management.Secondary research encompassed regulatory guidelines, industry standards, and open-source trade and policy records relevant to cross-border tariffs and supply chain shifts. Data from multiple sources were cross-validated, and thematic analysis identified consistent patterns around automation adoption, reagent standardization, and service economics. Scenario analysis addressed tariff-driven supply chain contingencies and regional differences in regulatory requirements. Throughout the methodology, emphasis was placed on reproducibility and transparency: assumptions were documented, interview protocols standardized, and findings were reviewed by domain experts and laboratory stakeholders to validate applicability and accuracy.
Conclusive synthesis highlighting how validated reproducibility, automation, and resilient support ecosystems will determine success in the evolving slide stainer landscape
In conclusion, the slide stainer landscape is maturing into a domain where reproducible staining, automation, and integrated service ecosystems determine competitive positioning and operational efficiency. Laboratories are increasingly evaluating solutions not merely on instrument throughput but on how reliably staining protocols interface with digital pathology, reagent continuity, and service responsiveness. Tariff shifts and supply chain fragility have added urgency to regionalization and localized support strategies, nudging both vendors and purchasers toward flexible contractual arrangements and contingency planning.Looking ahead, organizations that combine technological innovation with pragmatic service designs and supply chain diversification will be best positioned to meet evolving clinical and research needs. The critical determinants of success will include validated reagent-instrument interoperability, responsive aftermarket support, and a clear path to integrating staining outputs with downstream digital analysis. This conclusion underscores the need for collaborative planning between manufacturers, laboratory leaders, and procurement teams to translate operational insights into durable improvements in diagnostic quality and throughput.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Slide Stainer Market
Companies Mentioned
The key companies profiled in this Slide Stainer market report include:- Agar Scientific Ltd.
- Agfa-Gevaert N.V.
- Agilent Technologies, Inc.
- Avantor, Inc.
- Beijing YIDI Medical Equipment Co., Ltd.
- Bio-Optica Milano S.p.A.
- BioGenex Laboratories, Inc.
- Boekeler Instruments, Inc.
- Bruker Corporation
- Carl Zeiss AG
- Clarapath, Inc.
- Danaher Corporation
- Diapath S.p.A.
- F. Hoffmann-La Roche Ltd
- Glatt GmbH
- Hologic, Inc.
- MEDITE GmbH
- Milestone S.r.l.
- Olympus Corporation
- PerkinElmer, Inc.
- Sakura Finetek U.S.A., Inc.
- SHANDONG TIANLI ENERGY CO., LTD.
- SLEE Medical GmbH
- Sysmex Corporation
- The Menarini Group
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
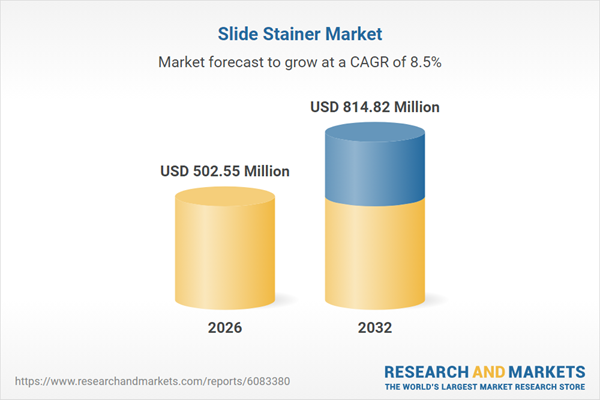
| Estimated Market Value ( USD | $ 502.55 Million |
| Forecasted Market Value ( USD | $ 814.82 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |