Speak directly to the analyst to clarify any post sales queries you may have.
Senior healthcare leaders face a dynamic landscape in the raloxifene hydrochloride market, driven by shifting therapeutic priorities, regulatory changes, and supply chain complexity. Understanding the multifactorial environment shaping clinical and commercial outcomes is essential for strategic decision-making.
Market Snapshot
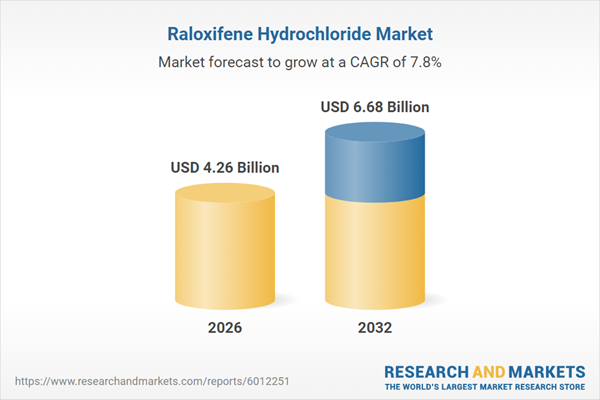
The Raloxifene Hydrochloride Market grew from USD 3.95 billion in 2025 to USD 4.26 billion in 2026. It is projected to advance at a CAGR of 7.79%, reaching USD 6.68 billion by 2032. Market expansion is propelled by evolving clinical guidelines, regulatory developments, and ongoing innovation in product accessibility across regions and channels.
Scope & Segmentation
- Clinical Indications: Breast Cancer Risk Reduction and Osteoporosis Prevention, each requiring tailored patient management strategies, differentiated engagement models, and unique monitoring protocols.
- Product Types: Branded and Generic, reflecting varying lifecycle management, support services, pricing pressures, and formulary emphasis.
- Distribution Channels: Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy, with logistics, patient support, and regulatory challenges specific to each channel.
- End-user Settings: Clinics, Homecare, and Hospitals, where prescribing routines, care coordination, and reimbursement mechanisms shape deployment and access strategies.
- Regions Covered: Americas, Europe Middle East & Africa, and Asia-Pacific, capturing differences in regulatory oversight, healthcare infrastructure, and commercialization practices.
- Technology Themes: Integration of digital health solutions for remote adherence monitoring and emerging manufacturing innovations supporting generic entry.
Key Takeaways
- Raloxifene hydrochloride occupies a unique position at the intersection of oncology prevention and bone health, offering dual benefit profiles for select high-risk populations and postmenopausal individuals.
- Therapeutic use and adoption are shaped by robust safety monitoring, postmarketing surveillance, and periodic updates to clinical practice guidelines driven by new evidence and real-world outcomes.
- Manufacturers face intensifying global regulatory scrutiny, particularly around quality controls for generics, while increasing digital engagement transforms supply chain management and patient adherence.
- Stakeholders must respond to shifting competitive dynamics, emphasizing differentiated patient support, targeted evidence generation, and agile lifecycle management to sustain relevance across branded and generic segments.
- Adaptation to region-specific challenges—such as regulatory fragmentation in EMEA, rapidly evolving healthcare modernization in Asia-Pacific, and value-based procurement in the Americas—is critical for successful market entry and sustained growth.
Tariff Impact and Supply Chain Strategies
Recent U.S. tariff adjustments for 2025 are increasing complexity for pharmaceutical supply chains. These changes influence cost structures on active ingredients, intermediate sourcing, and finished products, prompting companies to review sourcing, elevate domestic production, and diversify supplier bases. Manufacturers are also adapting inventory planning and renegotiating contracts to manage border policy risks and ensure operational resilience. For generics, these pressures may drive consolidation or vertical integration, while branded sponsors could adjust pricing or support strategies to maintain market traction. Downstream, payers are recalibrating procurement criteria, affecting distribution outcomes and patient access.
Methodology & Data Sources
This report utilizes a robust, multi-method research approach. Structured interviews with clinical leaders and procurement experts provide primary insights. Secondary data include peer-reviewed studies, regulatory filings, clinical trials, and patent records. Analytical measures encompass guideline synthesis, supply chain scenario analysis, and expert cross-validation. All findings are rigorously verified and documented to support decision-makers in clinical, commercial, and policy functions.
Why This Report Matters
- Equips leaders with a transparent view of clinical, operational, and market-access drivers impacting raloxifene hydrochloride adoption across varied geographies.
- Clarifies how evolving evidence, tariff-driven cost shifts, and segment-specific engagement models inform both short- and long-term commercial strategies.
- Enables the alignment of evidence generation, supply resilience, and stakeholder collaboration to ensure continued patient access and competitive positioning.
Conclusion
Raloxifene hydrochloride’s market future relies on integrating clinical innovation, operational resilience, and adaptive access models. Stakeholders leveraging targeted research and flexible strategies will maintain relevance and support sustained patient outcomes in a dynamic landscape.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Raloxifene Hydrochloride Market
Companies Mentioned
The key companies profiled in this Raloxifene Hydrochloride market report include:- Amneal Pharmaceuticals LLC
- Aurobindo Pharma Limited
- Cadila Pharmaceuticals Ltd.
- Camber Pharmaceuticals, Inc.
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- Eli Lilly and Company
- Enzo Biochem, Inc.
- Glenmark Pharmaceuticals Ltd.
- Glochem Industries Pvt. Ltd.
- Guangzhou Tosun Pharmaceutical Co., Ltd.
- Healthy Inc.
- Medilux Laboratories Pvt. Ltd.
- Novique Life Sciences Pvt. Ltd.
- Sanika Chemical Pvt. Ltd.
- ScieGen Pharmaceuticals, Inc.
- Shreeji Pharma International
- Srini Pharmaceuticals Pvt. Ltd.
- Taj Pharmaceuticals Limited
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 4.26 Billion |
| Forecasted Market Value ( USD | $ 6.68 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |