Speak directly to the analyst to clarify any post sales queries you may have.
Strategic overview that situates nucleic acid therapeutics within the modern innovation landscape and highlights the critical translational drivers
Nucleic acid-based therapeutics are redefining the boundaries of modern medicine by leveraging precise genetic and molecular mechanisms to treat a wide spectrum of diseases. Over the past decade, advances in oligonucleotide chemistry, delivery vectors, and manufacturing techniques have moved what were once experimental concepts into clinical reality, enabling targeted modulation of gene expression and protein synthesis. As a result, researchers and development teams are increasingly prioritizing modality-specific optimization, such as backbone stabilization, sequence design for off-target minimization, and advanced lipid or polymeric delivery systems that enhance tissue selectivity and biostability.Concurrently, regulatory frameworks are adapting to accommodate the unique attributes of these modalities, with agencies offering more flexible pathways for accelerated development when robust mechanistic rationale and biomarker strategies are present. Investment patterns reflect heightened interest from both established biopharma and specialized biotech ventures, with collaborations emerging to bridge expertise in chemistry, delivery, and clinical translation. This introductory overview provides context for the detailed analyses that follow, emphasizing the technological, regulatory, and commercial forces shaping translational trajectories and the strategic choices organizations must make to convert promising science into viable therapeutics.
How technological breakthroughs, delivery innovations, and evolving regulatory frameworks are jointly reshaping development pathways and market dynamics
The landscape for nucleic acid-based drugs is experiencing transformative shifts driven by technological breakthroughs, evolving delivery paradigms, and a maturation of regulatory approaches. Advances in messenger RNA engineering and synthetic stabilization chemistries have expanded the therapeutic possibilities beyond traditional antisense and RNA interference approaches, while next-generation delivery technologies are beginning to resolve long-standing challenges related to tissue targeting and immune activation. In parallel, integration of high-throughput screening, machine learning guided sequence optimization, and refined pharmacokinetic modeling has accelerated candidate prioritization and reduced attrition in early development.Moreover, the ecosystem is witnessing a redistribution of capabilities through strategic partnerships and service networks that combine discovery expertise with scalable manufacturing. Investment in in-house and outsourced manufacturing capacity for nucleic acid substrates and lipid nanoparticle formulation is increasing, creating more resilient supply chains and enabling faster clinical translation. Regulatory bodies are also clarifying expectations around quality attributes, comparability, and safety monitoring for these novel modalities, which in turn influences development timelines and risk management approaches. Together, these shifts are reshaping competitive dynamics and opening new therapeutic windows for precision interventions across diverse disease areas.
Assessing how recent trade measures and tariff dynamics are prompting procurement realignments, supply chain resilience planning, and manufacturing localization strategies
Recent tariff policies and trade measures originating in the United States have introduced additional complexity into the operational calculus for developers, manufacturers, and suppliers of nucleic acid therapeutics. Tariff-driven cost pressures affect inputs such as specialized raw materials, proprietary nucleotides, lipid excipients, and certain manufacturing equipment, which can increase procurement lead times and incentivize reconfiguration of supplier networks. As a consequence, many organizations are re-evaluating sourcing strategies, exploring dual-sourcing options, and prioritizing suppliers located within tariff-favored jurisdictions to mitigate exposure to import duties and logistical unpredictability.Furthermore, tariffs interact with regulatory and quality considerations in ways that complicate supply continuity. For instance, switching suppliers to avoid tariff impacts may require additional validation, comparability assessments, and regulatory notifications, thereby introducing potential delays. From an operational perspective, companies are increasingly building scenario-based procurement playbooks that account for tariff fluctuations, customs compliance, and inventory optimization to preserve clinical and commercial timelines. In the strategic domain, some stakeholders are accelerating investments in local or nearshore manufacturing capabilities to reduce dependency on cross-border flows, while others are leveraging contractual hedging and long-term supply agreements to absorb short-term cost volatility without disrupting development programs.
Integrated segmentation analysis detailing modality, molecule, delivery route, therapeutic focus, and end-user implications to guide development and commercial choices
Segmentation insights reveal modality-specific considerations that influence development strategies and commercial positioning across drug type, molecule type, route of administration, therapeutic area, and end-user. When analyzed by drug type, distinctions among antisense oligonucleotides, DNA/RNA aptamers, mRNA-based therapeutics, nucleoside analogs, and RNA interference approaches highlight differences in chemical optimization, delivery needs, and safety monitoring; within RNA interference, the submodalities microRNA, short hairpin RNA, and short interfering RNA each present unique potency and duration trade-offs that inform target selection and dosing strategies. Considering molecule type, the contrast between large molecule and small molecule modalities affects manufacturing platforms, cold chain logistics, and regulatory dossier content, prompting varied capital investments and operational footprints.Route of administration shapes clinical trial design and patient adoption pathways, as inhalation, intramuscular, intravenous, oral, and subcutaneous delivery routes carry distinct formulation and device requirements; decisions here impact patient compliance, distribution networks, and pharmacovigilance approaches. Therapeutic area segmentation, including cardiovascular, infectious, metabolic, neurological, oncology, and rare diseases, further refines development priorities: infectious disease applications require pathogen-specific delivery and rapid response capabilities, with bacterial, fungal, and viral subcategories each demanding specialized preclinical models, while oncology programs must reconcile distinctions between hematologic malignancies and solid tumors in terms of tumor microenvironment penetration and biomarker-driven patient selection. Finally, end-user segmentation across academic and research institutes, contract research organizations, hospitals and clinics, and pharmaceutical and biotechnology companies indicates differentiated demand for services, assay capabilities, and scalability support, shaping how commercial and collaborative models are structured in the value chain.
Comparative regional dynamics and operational implications that influence clinical development, manufacturing siting, and market access strategies across three global clusters
Regional dynamics exert a strong influence on regulatory approaches, talent availability, reimbursement pathways, and manufacturing infrastructure across the Americas, Europe Middle East and Africa, and Asia Pacific. In the Americas, policy environments and large private investment pools have fostered innovation hubs where translational science moves rapidly toward clinical testing, benefiting from established venture ecosystems and integrated clinical networks. Conversely, Europe Middle East and Africa present a heterogeneous regulatory landscape that combines advanced regulatory science in certain jurisdictions with emerging capacities in others, creating both partnership opportunities and harmonization challenges for cross-border clinical programs. In the Asia Pacific region, fast-growing technical capabilities, expansive manufacturing scale, and pronounced government support in some countries are enabling rapid expansion of production capacity and collaborative R&D models that cater to both regional and global demand.These regional distinctions influence strategic decisions about clinical trial geographies, manufacturing siting, and market entry sequencing. Stakeholders frequently adopt a differentiated approach that leverages regional strengths-such as trial patient recruitment efficiency in certain markets, cost-effective manufacturing nodes in others, and regulatory expertise concentrated in specific jurisdictions-to optimize timelines and resource allocation. As regulatory convergence efforts progress and cross-border collaboration increases, companies that align regional operational footprints with clinical and commercial strategies will realize more resilient pathways to development and patient access.
How platform innovators, delivery specialists, and manufacturing partners collaborate and differentiate to accelerate translation and secure competitive advantage
Key companies in the nucleic acid therapeutics ecosystem are distinguished by their capabilities across discovery platforms, delivery technologies, manufacturing scale-up, and regulatory execution. Some organizations specialize in proprietary chemistry and sequence optimization tools that accelerate candidate selection, while others focus on delivery vehicles and formulation innovations that address biodistribution and immunogenicity constraints. Manufacturing-focused enterprises offer GMP-compliant nucleotide synthesis, lipid nanoparticle production, and fill-finish services, forming critical pillars for de-risking late-stage programs. Collaboration models are increasingly prevalent, where technology licensors, contract development and manufacturing providers, and clinical sponsors coalesce to aggregate complementary expertise and share development risk.Competitive differentiation often emerges from integrated capabilities that span platform innovation to commercial readiness, including demonstrated regulatory interactions and successful bridging studies that validate comparability after process changes. In addition, companies that invest in modular, scalable manufacturing and robust quality systems are better positioned to support rapid clinical expansion and commercial roll-out. Strategic partnerships between platform owners and end-to-end service providers continue to redefine market dynamics, enabling therapeutics to move efficiently from bench to bedside while allocating capital to the highest-value activities across the development continuum.
Actionable strategic recommendations for aligning scientific innovation, supply chain resilience, and regulatory engagement to accelerate therapeutic translation and market readiness
Industry leaders should adopt an integrated strategy that aligns platform science, delivery solutions, and operational resilience to accelerate development and commercialization of nucleic acid therapies. First, prioritize investment in delivery science and formulation platforms that demonstrably improve tissue targeting and reduce systemic exposure, while concurrently building rigorous safety and immunogenicity monitoring frameworks. Second, diversify supplier relationships for critical reagents and components and implement comparability pathways in advance so that supplier transitions do not jeopardize regulatory continuity or trial timelines. Third, cultivate strategic partnerships that combine discovery strengths with manufacturing scale and regulatory expertise to shorten development cycles and share technical risks.Additionally, organizations should proactively engage with regulatory authorities to define acceptable quality attributes and adaptive development plans, leveraging scientific advice to streamline dossier preparation. Build modular manufacturing capacity or secure partnerships with flexible contract manufacturers to enable rapid scale-up and mitigate tariff-induced disruptions. Finally, invest in cross-functional teams that bridge biology, chemistry, regulatory, and commercial disciplines to ensure that early technical decisions are informed by downstream operational and market access considerations, thereby increasing the likelihood of successful translation and patient adoption.
Transparent and reproducible research approach combining expert consultations, literature synthesis, and scenario analysis to support strategic decision making
The research methodology underpinning this analysis combines a systematic review of scientific literature, regulatory guidance, and industry disclosures with qualitative interviews and triangulated validation from subject matter experts across discovery, manufacturing, and regulatory domains. Primary qualitative inputs were obtained through structured consultations with leaders in oligonucleotide chemistry, delivery engineering, GMP manufacturing, and regulatory affairs to capture nuanced operational realities and evolving best practices. Secondary sources included peer-reviewed publications, agency guidance documents, and company technical disclosures to provide a robust evidence base for modality-specific insights.Synthesis of findings emphasized cross-validation between expert perspectives and publicly available technical materials, ensuring that conclusions are grounded in observable technological trends and documented regulatory developments. The methodology also incorporated scenario analysis for supply chain and tariff-related contingencies to assess operational impacts and mitigation pathways. Throughout, attention was paid to reproducibility and transparency so that readers can trace analytic judgments back to the underlying evidence and adapt conclusions to their organizational context.
Concluding synthesis that connects technological promise with operational imperatives to guide stakeholders toward resilient and patient focused translation strategies
In conclusion, nucleic acid-based therapeutics occupy a pivotal position in the next wave of precision medicine, driven by advances in chemistry, delivery, and regulatory science that collectively lower barriers to clinical translation. Success in this domain depends on harmonizing scientific innovation with operational execution-particularly in delivery optimization, manufacturing scalability, and supply chain robustness. Institutions that integrate modality-specific expertise with pragmatic supply strategies and proactive regulatory engagement will be better positioned to convert early promise into safe, effective therapies that reach patients.Looking forward, the most resilient organizations will adopt flexible, partnership-oriented operating models that can absorb external shocks such as trade-driven cost pressures while maintaining clinical momentum. By aligning technical choices with end-to-end commercialization considerations, stakeholders can navigate complexity more effectively and accelerate the adoption of nucleic acid therapeutics across a wide range of unmet medical needs.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Nucleic Acid-Based Drugs Market
Companies Mentioned
The key companies profiled in this Nucleic Acid-Based Drugs market report include:- Alnylam Pharmaceuticals, Inc.
- Amgen Inc.
- Arcturus Therapeutics Holdings Inc.
- Arrowhead Pharmaceuticals, Inc.
- AstraZeneca PLC
- Beam Therapeutics Inc.
- Biogen, Inc.
- BioMarin Pharmaceutical Inc.
- BioNTech SE
- Bluebird Bio, Inc.
- CRISPR Therapeutics AG
- CureVac N.V.
- Dynavax Technologies Corporation
- Editas Medicine, Inc.
- Eli Lilly and Company
- Evotec SE
- F. Hoffmann-La Roche Ltd.
- Generation Bio Co.
- Gilead Sciences, Inc.
- GSK PLC
- Intellia Therapeutics, Inc.
- Ionis Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Moderna, Inc.
- Novartis AG
- Novo Nordisk A/S
- Orna Therapeutics, Inc.
- Pfizer Inc.
- ProQR Therapeutics N.V.
- Sangamo Therapeutics, Inc.
- Sanofi SA
- Sarepta Therapeutics, Inc.
- Silence Therapeutics PLC
- Stoke Therapeutics, Inc.
- Takeda Pharmaceutical Company Limited
- Vertex Pharmaceuticals Incorporated
- Voyager Therapeutics, Inc.
- Wave Life Sciences Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
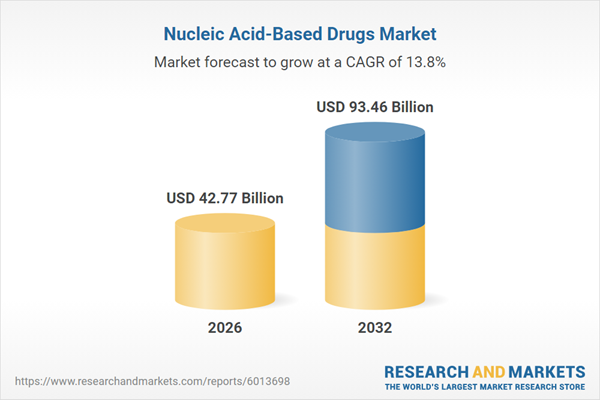
| Estimated Market Value ( USD | $ 42.77 Billion |
| Forecasted Market Value ( USD | $ 93.46 Billion |
| Compound Annual Growth Rate | 13.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 39 |