Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction framing benexate’s therapeutic relevance, regulatory context, formulation considerations, and stakeholder implications for clinical and commercial teams
Benexate occupies a distinct therapeutic niche with implications across clinical practice, regulatory oversight, and commercial strategy. Its pharmacologic profile and therapeutic applications invite consideration from multiple clinical stakeholders, while formulation options and routes of administration shape clinical utility and patient adherence. In recent years, attention has shifted toward optimizing delivery formats and aligning regulatory submissions with evolving safety and efficacy expectations, prompting developers and commercialization teams to reconsider development timelines and go-to-market tactics.Clinicians prioritize predictable therapeutic action and manageable safety profiles, which in turn influence prescribing patterns and formulary decisions. Concurrently, regulatory authorities are increasingly focused on post-market surveillance and real-world evidence, creating both obligations and opportunities for manufacturers to demonstrate product value beyond pivotal trials. Meanwhile, payers and procurement stakeholders are influenced by distribution pathways and supply-chain resilience, which can affect availability across care settings from hospitals to home care. Taken together, these factors frame the competitive and operational landscape for benexate, requiring coordinated action across clinical development, regulatory strategy, and commercial planning to realize therapeutic and business objectives.
Transformative shifts in formulation innovation, regulatory emphasis on real-world evidence, and distribution evolution reshaping benexate development and access pathways
The landscape for benexate and comparable therapeutic agents has shifted as technology, policy, and clinical practice evolve. Advances in formulation science have enabled renewed focus on patient-centric delivery, with emphasis on oral suspensions and tablet optimization to support adherence in ambulatory and home-care environments. At the same time, injectables and intravenous administration remain essential for acute-care scenarios, underscoring the need for a portfolio approach that addresses diverse care pathways and patient acuity.Regulatory trends are driving greater demand for robust safety monitoring and real-world data generation, which reshapes lifecycle planning and post-approval commitments. Distribution models have also transformed; mail order and online pharmacy channels gained traction and persist as important access points alongside traditional hospital and retail pharmacies. The continued expansion of specialty clinics and ambulatory surgical centers into areas historically dominated by inpatient care has amplified demand for stable supply and predictable procurement channels. Together, these shifts compel organizations to revisit R&D priorities, commercial segmentation, and supply-chain redundancy to remain competitive and responsive to clinician and patient needs.
Cumulative effects of recent United States tariff changes on supply chains, sourcing strategies, and commercial cost management for benexate and associated formulations
Recent tariff adjustments originating from United States trade policy have exerted measurable pressure on cost structures for imported active pharmaceutical ingredients and finished formulations. Supply-chain stakeholders have had to reassess sourcing strategies, with an increased focus on supplier diversification and the validation of domestic or near-shore alternatives to mitigate exposure to import duties. Pharmaceutical firms have responded by accelerating qualification of secondary suppliers, reevaluating contractual terms, and increasing inventory buffers to protect continuity of supply for both acute-care injectable lines and chronic outpatient formulations.These trade-related shifts also influence pricing negotiations with payers and procurement entities, who may demand transparency on cost drivers and expect manufacturers to absorb or justify incremental cost inputs. Manufacturers face a strategic choice between absorbing short-term tariff impacts to maintain competitive access or pursuing operational adjustments that could include regionalized manufacturing, changes to packaging or batch consolidation, and optimized freight strategies. In practice, organizations that proactively address tariff-induced disruptions through supplier qualification, logistics optimization, and stakeholder communication preserve market access and protect clinical continuity across hospitals, specialty clinics, and home-care settings.
Key segmentation-driven commercial intelligence illustrating how formulation, administration route, prescription status, distribution channels, and end-user dynamics dictate strategy
Segmentation insights reveal how therapeutic use-cases and operational realities drive differentiated demand and strategic priorities. Based on dosage form, stakeholders evaluate opportunities across capsules, injectables, oral suspensions, and tablets, which in turn influence formulation development, stability studies, and packaging requirements. Based on route of administration, consideration of intravenous versus oral pathways informs clinical trial design, hospital formulary placement, and outpatient adherence strategies. Based on prescription status, the dichotomy between over-the-counter and prescription channels creates distinct regulatory pathways, promotional constraints, and payer interactions that must be navigated.Based on distribution channel, the spectrum from hospital pharmacy through mail order, online, and retail pharmacy segments requires tailored logistics, contracting, and channel-specific marketing approaches; within hospital pharmacy, secondary and tertiary facilities have unique procurement cycles and clinical committee structures that affect adoption, while chain and independent retail pharmacies differ in purchasing scale and local formulary influence. Based on end user, demand originates from ambulatory surgical centers, home care settings, hospitals, and specialty clinics; within specialty clinics, gastroenterology and general clinics present varied prescribing patterns and clinical workflows that shape training, sampling, and support programs. Synthesizing these segmentation lenses enables companies to prioritize development investments, align promotional efforts with clinical touchpoints, and optimize distribution to match the point-of-care realities that determine product use.
Regional insights across the Americas, Europe Middle East & Africa, and Asia-Pacific highlighting regulatory diversity, supply-chain considerations, and channel strategies for benexate
Regional dynamics shape regulatory navigation, supply-chain decisions, and commercialization tactics for benexate across major global blocks. In the Americas, pricing pressure and payer scrutiny encourage evidence generation and targeted access strategies that emphasize hospital tender processes and retail distribution resilience. Manufacturers in this region often prioritize partnerships with contract manufacturers and logistics providers to ensure timely supply to both acute-care and outpatient channels.In Europe, Middle East & Africa, regulatory heterogeneity and fragmented procurement ecosystems require nuanced country-level regulatory engagement and adaptive pricing tactics to accommodate diverse reimbursement systems and variable clinical adoption patterns. Stakeholders in this region frequently invest in local regulatory expertise and regional distribution agreements to bridge access gaps. In the Asia-Pacific region, rapid expansion of outpatient care and digital pharmacy channels creates opportunities for scalable distribution and patient support programs, while supply-chain advantages in manufacturing capacity and raw-material sourcing invite strategic sourcing and regional production strategies. Across all regions, a combination of regulatory agility, supply-chain redundancy, and channel-specific commercialization drives successful market entry and sustained product availability.
Competitive company insights focusing on formulation capabilities, manufacturing flexibility, distribution partnerships, and evidence-generation strategies driving benexate leadership
Competitive dynamics are influenced by differentiated capabilities across clinical development, manufacturing scale, and channel relationships. Leading organizations demonstrate strength in advancing diverse dosage forms while managing the regulatory complexity of transitioning between prescription and over-the-counter models. Companies that maintain flexible manufacturing networks and invest in formulation science are better positioned to supply both injectable portfolios for acute settings and oral alternatives for ambulatory care.Partnerships and alliances with contract manufacturing organizations, specialty distributors, and digital pharmacy platforms are increasingly important levers for accelerating access and expanding reach. Firms that pair clinical development with robust post-market surveillance and real-world evidence programs secure stronger payer engagement and clinician confidence. Strategic licensing, co-promotion, and targeted academic collaborations remain vital mechanisms for extending therapeutic indications and establishing clinician familiarity across gastroenterology and general practice settings, thereby reinforcing prescribing continuity across care environments.
Actionable recommendations for industry leaders to align formulation strategy, supply-chain resilience, evidence generation, and distribution optimization to seize clinical and commercial advantage
Industry leaders should adopt integrated strategies that align clinical development, regulatory planning, and commercial execution. First, accelerate formulation development that prioritizes patient adherence across both oral and injectable options to support adoption in ambulatory and acute settings. Second, strengthen supplier diversification and validate contingency manufacturing to reduce tariff exposure and logistical bottlenecks; near-shore or regionalized production can materially improve supply reliability while enhancing responsiveness to local regulatory requirements.Third, invest in real-world evidence initiatives that demonstrate safety and comparative clinical utility across care settings, enabling stronger payer conversations and streamlined formulary inclusion. Fourth, tailor distribution and channel strategies to the nuanced needs of hospitals, specialty clinics, retail pharmacies, and evolving online platforms, ensuring that procurement processes and clinical workflows are accommodated. Finally, pursue strategic partnerships with contract manufacturers, distributors, and specialty clinics to expand reach while controlling capital intensity, and align commercial messaging to clinical stakeholders by leveraging targeted medical education and outcomes data to drive sustainable adoption.
Research methodology outlining primary interviews, regulatory review, distribution intelligence, and a segmentation-first analytical framework used to derive actionable insights
This research synthesized primary interviews with clinical experts, procurement leaders, and supply-chain operators, together with a structured review of regulatory guidance documents, publicly available clinical literature, and distribution channel reports. Data collection emphasized triangulation: qualitative inputs from key opinion leaders were cross-referenced with operational intelligence from distribution partners and regulatory filings to ensure balanced interpretation. Where proprietary retrospective datasets were used, they were corroborated against independent sources and contextualized within prevailing policy developments to maintain analytic rigor.Analysts applied a segmentation-first approach to evaluate clinical use-cases, distribution pathways, and end-user behavior by mapping dosage form, route of administration, prescription status, distribution channel, and end-user type to observed adoption patterns and procurement mechanisms. Regional analysis incorporated regulatory frameworks, supply-chain footprints, and channel maturity to identify differential pathways to access. Findings reflect synthesis across qualitative and quantitative inputs and prioritize clarity for decision-makers seeking to translate insight into operational action.
Concluding synthesis emphasizing the need to integrate formulation diversity, evidence generation, and resilient supply-chain strategies to secure clinical and commercial success for benexate
In conclusion, benexate’s strategic potential rests on the ability of stakeholders to align formulation diversity, regulatory strategy, and supply-chain resilience with evolving distribution channels and care pathways. Clinical utility spans both oral and intravenous administration, which requires a coordinated approach to development, evidence generation, and promotional alignment with clinicians across hospitals, specialty clinics, and home-care environments. At the same time, trade-policy adjustments and channel evolution demand proactive sourcing, logistics optimization, and regional manufacturing considerations to maintain uninterrupted access.Organizations that prioritize patient-centric formulation, invest in real-world evidence, and secure flexible manufacturing and distribution partnerships will be best positioned to navigate the changing landscape. Ultimately, converting insight into competitive advantage requires integrating scientific rigor with commercial pragmatism and operational agility to meet clinician needs and sustain product availability across the full spectrum of care.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Benexate Market
Companies Mentioned
The key companies profiled in this Benexate market report include:- Astellas Pharma Inc.
- Chugai Pharmaceutical Co., Ltd.
- Daiichi Sankyo Company, Limited
- Dr. Reddy’s Laboratories Limited
- Eisai Co., Ltd.
- Glenmark Pharmaceuticals Ltd.
- Kyowa Kirin Co., Ltd.
- Meiji Seika Pharma Co., Ltd.
- Mitsubishi Tanabe Pharma Corporation
- Natco Pharma Limited
- Nichi-Iko Pharmaceutical Co., Ltd.
- Nipro Corporation
- Otsuka Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Sandoz International GmbH
- Sawai Pharmaceutical Co., Ltd.
- Shionogi & Co., Ltd.
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Teijin Pharma Limited
- Teva Pharmaceutical Industries Ltd.
- Towa Pharmaceutical Co., Ltd.
- Viatris Inc.
- Wooshin Labottacah Co., Ltd.
- Zibo Hangyu Biotechnology Development Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
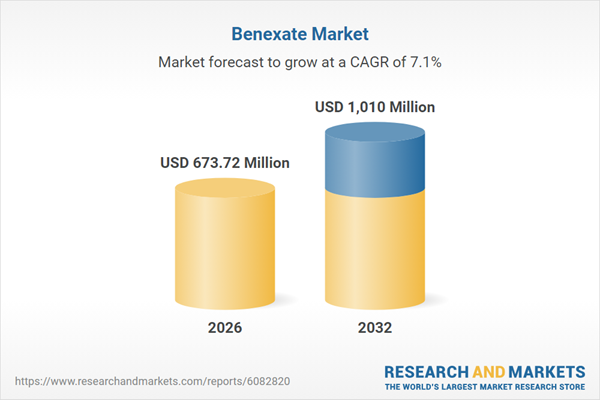
| Estimated Market Value ( USD | $ 673.72 Million |
| Forecasted Market Value ( USD | $ 1010 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |