Speak directly to the analyst to clarify any post sales queries you may have.
An illuminating introduction to four-dimensional imaging that contextualizes its technological advances, clinical utility, and operational implications for healthcare systems
Four-dimensional imaging is transitioning from an experimental capability to an integral clinical tool that augments diagnosis, interventional planning, and longitudinal patient monitoring. Early adopters in tertiary care centers have demonstrated how temporal resolution combined with spatial detail can reveal physiologic dynamics that static images cannot, enabling clinicians to analyze blood flow, organ motion, and functional connectivity with greater granularity. This evolution is driven by parallel advances in detector technology, reconstruction algorithms, and real-time visualization platforms that together produce datasets rich in clinical signals.As these capabilities have matured, the ecosystem has expanded to include not only established imaging modalities such as computed tomography, magnetic resonance imaging, and ultrasound but also integrated software and cloud-native services that facilitate multi-dimensional analysis. Consequently, operational workflows are adapting; radiology teams are redefining protocols to include dynamic acquisitions and multidisciplinary teams are devising new interpretation pathways. These shifts are prompting healthcare providers to re-evaluate equipment procurement, staff training, and data governance practices to fully harness the diagnostic and therapeutic potential of four-dimensional imaging.
Taken together, the introduction of four-dimensional imaging represents a pivotal inflection point for clinical imaging. It demands coordinated investments across technology, personnel, and informatics to convert enhanced imaging fidelity into measurable improvements in patient care, throughput, and clinician confidence.
How accelerated hardware innovation, intelligent reconstruction, and integrated software ecosystems are redefining clinical workflows and competitive dynamics in medical imaging
The landscape of clinical imaging is undergoing transformative shifts driven by innovations in acquisition speed, reconstruction intelligence, and interoperability. Rapid improvements in hardware design, including high-throughput detectors and specialized transducers, have reduced motion artifacts and enabled dynamic studies that were previously impractical in routine clinical settings. Simultaneously, machine learning-driven reconstruction and denoising algorithms are enhancing temporal fidelity while minimizing radiation exposure and scan time, thereby making dynamic imaging more acceptable across patient populations.Interoperability has emerged as a defining trend, with software platforms increasingly capable of integrating four-dimensional datasets into existing PACS and workflow engines. This integration facilitates multimodal correlation and longitudinal tracking, allowing clinicians to compare dynamic physiological metrics across time and therapy. In parallel, regulatory frameworks and clinical validation pathways are evolving to accommodate device-software combinations, which is reshaping how vendors approach product development and clinical trials.
Moreover, care delivery models are adapting: cardiology and neurology services are incorporating dynamic metrics into routine decision-making, while ambulatory and outpatient centers are beginning to adopt hybrid deployment models that combine local acquisition with cloud-based post-processing. These shifts are creating new competitive dynamics between traditional imaging vendors, software specialists, and clinical service providers, accelerating the pace at which four-dimensional imaging becomes a standard component of diagnostic and therapeutic care.
Assessing the immediate and strategic repercussions of 2025 United States tariff adjustments on supply chains, procurement decisions, and vendor product strategies in imaging
In 2025, tariff policies across the United States introduced elevated duties on a range of imported medical imaging components and finished systems, affecting supply chain economics and vendor strategies. These trade measures have led device manufacturers and system integrators to re-evaluate sourcing strategies for scanners, transducers, and precision electronics that are central to four-dimensional imaging solutions. In response, many suppliers accelerated supply chain diversification efforts to mitigate exposure to tariff-induced cost volatility, seeking alternate manufacturing locations and regional distribution hubs.The tariff environment also influenced procurement timelines for health systems. Capital projects that involved high-cost imaging suites experienced extended negotiations as buyers assessed total cost of ownership under the new import duty structure. Some institutions deferred non-urgent upgrades, while others prioritized domestic-sourced or locally assembled components where feasible. At the same time, vendors offering modular or software-centric solutions that could retrofit existing hardware found increased interest, since these options presented lower immediate import sensitivity and shorter deployment timelines.
In conclusion, the tariff changes prompted a broad reassessment of procurement, manufacturing, and product strategy within the four-dimensional imaging value chain. The near-term effect catalyzed localization and modularization trends, while the medium-term consequence is likely to be a more geographically distributed manufacturing footprint and evolving vendor propositions that emphasize software-enabled upgrades and services to preserve continuity of clinical innovation.
Comprehensive segmentation insights revealing how modality capabilities, clinical applications, end-user profiles, component ecosystems, and deployment models shape adoption strategies for four-dimensional imaging
A rigorous segmentation framework clarifies where four-dimensional imaging is creating clinical and commercial traction and which subsegments require differentiated strategies. By technology, computed tomography, magnetic resonance imaging, and ultrasound each present distinct value propositions and technical constraints. Computed tomography pathways focus on dynamic angiography, perfusion studies, and volumetric assessments that benefit from rapid gantry rotation and advanced reconstruction. Magnetic resonance workflows emphasize flow-sensitive sequences, functional mapping, and perfusion protocols that demand high temporal resolution and sophisticated post-processing. Ultrasound applications capitalize on real-time frame rates for echocardiography, fetal imaging, musculoskeletal assessments, and vascular evaluations where portability and operator skill influence adoption.Applications define clinical prioritization, with cardiology leveraging blood flow analysis and valve assessment to inform interventions, neurology adopting brain mapping and stroke evaluation for acute decision-making, obstetrics and gynecology using dynamic fetal monitoring and placental assessment to enhance perinatal outcomes, oncology applying response monitoring and treatment planning for adaptive therapies, and orthopedics using joint movement analysis and spine assessment to inform surgical planning and rehabilitation pathways. These clinical use cases inform imaging protocols and downstream analytics requirements.
End-user segmentation highlights deployment realities across ambulatory surgical centers with focused cardiac and orthopedic suites, diagnostic centers emphasizing throughput in imaging centers and outpatient clinics, hospitals that balance general, specialty, and academic demands, and research institutes that drive methodological innovation. Component segmentation underscores the interdependence of gantry and scanner hardware, monitoring systems, transducers and probes, workstations, consulting and maintenance services, and software capabilities such as analytics, image processing, integration with information systems, and advanced visualization. Finally, deployment models-cloud, hybrid, and on premise-present trade-offs between scalability, latency, and data governance, with private cloud and public cloud options, edge computing and multicloud integrations, and local infrastructure or private data center configurations shaping adoption pathways and vendor offerings.
Regional adoption patterns and infrastructure dynamics explaining how the Americas, EMEA, and Asia-Pacific uniquely influence the deployment and evolution of four-dimensional imaging
Regional dynamics are shaping the pace and pattern of adoption for four-dimensional imaging, with each geography presenting distinct clinical priorities, regulatory pathways, and infrastructure realities. In the Americas, advanced hospital networks and specialty centers are early adopters, driven by clinical demand for high-resolution cardiac and neurovascular diagnostics, a mature capital equipment market, and a strong private payer presence that incentivizes outcomes-oriented investments. This environment accelerates integration of dynamic imaging into interventional planning and value-based care initiatives, while also supporting robust vendor partnerships and domestic manufacturing adjustments influenced by recent trade policies.Across Europe, the Middle East, and Africa, diverse regulatory regimes and variable healthcare infrastructure create a heterogeneous adoption landscape. Western Europe demonstrates steady uptake within tertiary care and academic centers, often supported by national research collaborations and centralized procurement practices. In contrast, markets within the Middle East and Africa show selective investments focused on centers of excellence and medical tourism hubs where high-acuity cardiac and oncologic services justify advanced imaging suites. Regional interoperability initiatives and harmonized clinical protocols are important enablers that accelerate cross-border knowledge transfer and adoption.
The Asia-Pacific region presents a spectrum from highly advanced imaging ecosystems in select urban centers to rapidly growing demand in emerging healthcare markets. Large-scale capital investments, increasing clinical specialization, and a strong emphasis on scalable cloud and hybrid deployment models drive uptake. Local manufacturing capabilities and regional partnerships are also shaping supply chains and vendor strategies, enabling more cost-competitive solutions and faster time-to-market for both hardware and software innovations.
Insights on how leading vendors are aligning hardware innovation, clinical-grade software, and outcome-focused services to shape competitive advantage in dynamic imaging
Leading companies in the four-dimensional imaging ecosystem are differentiating along axes of modality specialization, software intelligence, and service-led offerings. Hardware manufacturers continue to invest in detector fidelity, motion compensation, and ergonomic designs that enable dynamic acquisitions across inpatient and ambulatory settings. At the same time, software vendors are focusing on advanced image processing, analytics, and visualization that translate temporal datasets into clinically interpretable metrics. These software capabilities often form the cornerstone of vendor value propositions, enabling retrospective analyses, faster reporting, and multidisciplinary collaboration.Service models are also evolving from traditional installation and maintenance contracts toward outcomes-oriented partnerships that incorporate training, performance optimization, and workflow redesign. Companies that combine strong clinical validation programs with practical deployment services can reduce adoption friction and accelerate hospital system buy-in. Research collaborations with academic centers and participation in multicenter clinical studies remain important differentiators, as they substantiate claims and support regulatory approvals for new dynamic imaging applications.
In summary, competitive positioning in this market depends on the ability to deliver integrated solutions that pair validated acquisition hardware with interoperable, clinically focused software and responsive service offerings. Firms that can demonstrate real-world impact on diagnostic confidence, procedural planning, or patient throughput are best positioned to lead adoption across diverse healthcare settings.
Actionable strategic recommendations for vendors and providers to accelerate clinical adoption, de-risk deployments, and maximize the value of dynamic imaging investments
Industry leaders seeking to capitalize on four-dimensional imaging should pursue a multifaceted strategy that aligns technical capability with clinical utility and operational feasibility. First, prioritize investments in cross-disciplinary validation studies that demonstrate how dynamic metrics improve diagnostic accuracy or procedural outcomes. These evidence-building activities should involve cardiology, neurology, obstetrics, oncology, and orthopedic stakeholders to ensure clinical relevance and to support reimbursement discussions. In parallel, accelerate development of interoperable software interfaces and APIs that enable seamless integration with existing imaging informatics and electronic health records, reducing adoption barriers for health systems that prioritize workflow continuity.Second, adopt modular product architectures and retrofit pathways that allow providers to upgrade existing scanners with dynamic-capable components or software, thereby preserving capital investments and expanding the addressable market. Complement these offerings with service packages that include targeted training, protocol optimization, and outcome monitoring to ensure clinical teams derive value quickly. Third, diversify manufacturing and distribution footprints to mitigate tariff risks and supply chain disruptions, while exploring regional partnerships to support localized service models.
Finally, prioritize transparent data governance and security practices for cloud and hybrid deployments to address institutional concerns about privacy and latency. Engage proactively with regulators and payers to align clinical evidence, coding, and reimbursement pathways. By executing on these recommendations, leaders can reduce friction, accelerate adoption, and create sustainable competitive moats based on clinical impact and operational excellence.
A transparent mixed-methods research approach combining clinician interviews, vendor briefings, technical literature, and policy analysis to underpin the report’s conclusions
This analysis synthesizes primary and secondary research methodologies to ensure rigor, reproducibility, and relevance for clinical and commercial decision-makers. Primary research included structured interviews and consultations with clinicians across cardiology, neurology, obstetrics, oncology, and orthopedics, as well as discussions with hospital procurement officers, imaging technologists, and research institute leads to validate clinical workflows and technology requirements. Vendor briefings and product demonstrations informed the technical assessment of acquisition hardware, transducer design, and software capabilities, while supply chain conversations illuminated manufacturing and distribution considerations.Secondary research encompassed a comprehensive review of peer-reviewed clinical literature, regulatory filings, technical white papers, and conference proceedings to map the trajectory of sequence development, reconstruction algorithms, and validation studies. Industry standards and interoperability specifications were examined to assess integration readiness, and public policy documents were analyzed to understand recent tariff actions and their implications for procurement. Data triangulation techniques were employed to reconcile divergent inputs and to construct a robust narrative around adoption drivers, barriers, and strategic responses.
Where uncertainties remained, sensitivity checks and expert adjudication were used to align interpretations with prevailing clinical practice. Throughout, ethical considerations and data privacy norms informed assessments of cloud and hybrid deployment viability. The methodology emphasizes transparency and traceability to support confident decision-making by technology buyers and strategic planners.
A concise conclusion synthesizing how technological convergence, clinical validation, and operational readiness will determine the impact of dynamic imaging on patient care
Four-dimensional imaging is positioned to become an influential enabler of precision diagnostics and personalized care pathways across multiple clinical domains. The convergence of faster acquisition hardware, intelligent reconstruction methods, and interoperable software platforms is transforming how clinicians perceive and utilize temporal physiological data. As the technology moves from early adopter centers into broader clinical practice, its greatest value will accrue where dynamic insights inform therapeutic choices, procedural planning, and longitudinal outcome assessment.Realizing this potential requires coordinated action across stakeholders: providers must adapt workflows and training, vendors must align product designs with integration and service expectations, regulators must clarify validation pathways, and payers must consider mechanisms to reward demonstrable improvements in care. Regional supply chain shifts and tariff developments underscore the need for resilient sourcing strategies and modular upgrade paths that protect institutional investments. Ultimately, the successful mainstreaming of four-dimensional imaging will be measured not only by installation counts but by its ability to change clinical decisions and improve patient outcomes in real-world settings.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China 4D Imaging in Healthcare Market
Companies Mentioned
- Agfa-Gevaert N.V.
- Analogic Corporation
- Arterys Inc.
- Canon Medical Systems Corporation
- Esaote S.p.A.
- Fujifilm Holdings Corporation
- General Electric Company
- Hitachi, Ltd.
- Konica Minolta Healthcare
- Koninklijke Philips N.V.
- Samsung Medison Co., Ltd.
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Siemens Healthineers AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
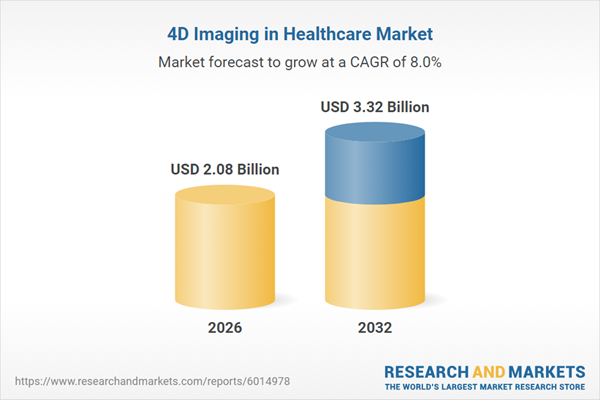
| Estimated Market Value ( USD | $ 2.08 Billion |
| Forecasted Market Value ( USD | $ 3.32 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |