Speak directly to the analyst to clarify any post sales queries you may have.
Framing the current clinical, scientific, and care delivery complexity in advanced recurrent ovarian cancer to inform strategic priorities and development focus
Advanced recurrent ovarian cancer occupies a complex clinical and scientific intersection that demands integrated thinking across therapeutics, diagnostics, and care delivery. Patients present with diverse molecular profiles and treatment histories that influence therapeutic sequencing, the selection of maintenance strategies, and supportive care needs. Clinicians are increasingly aligning systemic therapies with biomarker status while surgical teams reassess the role of cytoreduction and intraperitoneal approaches in recurrent settings. Simultaneously, payers and health systems are adapting pathways to accommodate precision diagnostics and high-cost oral and parenteral agents, creating a multidimensional environment for drug developers, device manufacturers, and service providers.Given this complexity, anyone entering into late-line development or commercial planning must synthesize evidence from randomized trials, real-world datasets, and evolving regulatory guidance. Bringing these threads together requires clear hypotheses about which patient subpopulations will benefit most, how delivery models can reduce time to therapy, and what diagnostics will be required to support label-concordant use. In short, this introduction frames advanced recurrent ovarian cancer as a field where scientific innovation, clinical pragmatism, and commercial foresight converge to shape impactful interventions and sustainable care pathways
How breakthroughs in targeted agents, diagnostics, and delivery models are redefining clinical strategy and patient selection in recurrent ovarian cancer
The treatment landscape for advanced recurrent ovarian cancer has shifted substantially with the maturation of targeted therapies, the growing precision of biomarker-driven decision-making, and the exploration of novel combination regimens. PARP inhibitors have reshaped maintenance paradigms for BRCA-mutant and homologous recombination-deficient patients, reinforcing the imperative for upfront genomic characterization. Parallel advances in anti-angiogenic agents and the refinement of cytotoxic backbones have created alternative maintenance and combination strategies, while antibody-drug conjugates and other novel modalities are entering late-phase development that may broaden therapeutic options beyond current classes.Concurrently, immunotherapy has demonstrated mixed results in ovarian cancer, prompting renewed focus on rational combinations, patient selection, and tumor microenvironment modulation rather than broad monotherapy adoption. Delivery innovations, including the expansion of oral targeted agents and ambulatory infusion models, are altering treatment adherence and resource utilization. Diagnostic evolution-particularly more sensitive and standardized HRD assays and circulating tumor DNA applications-has enabled earlier identification of actionable vulnerabilities and refined disease monitoring. Taken together, these transformative shifts create both opportunities and complexities for clinical program design, reimbursement strategy, and real-world adoption pathways
Evaluating how cumulative tariff and trade dynamics through 2025 may influence supply chain resilience, access pathways, and procurement strategies for oncology therapies
Policy and trade developments that affect pharmaceutical importation and supply chains can materially influence access dynamics for oncology therapies, particularly for complex biologics and specialized formulations. Cumulative tariff measures enacted through 2025 have the potential to increase procurement overheads for providers and distributors, generate greater variability in pricing across channels, and intensify pressure on manufacturers to localize certain aspects of production or distribution. In response, supply chain leaders and commercial teams are reassessing sourcing strategies, contracting terms, and inventory buffers to insulate patients and providers from episodic disruptions.The regulatory and reimbursement environment is also adapting; procurement stakeholders are placing greater emphasis on value demonstration and total cost of care when formulary decisions intersect with elevated import-related costs. Health systems may shift sourcing toward domestically produced generics or biosimilars where available, while manufacturers may explore nearshoring, additional contract manufacturing partnerships, or tiered pricing strategies to preserve channel continuity. For clinical programs, these dynamics reinforce the importance of early stakeholder engagement and contingency planning for drug supply to ensure uninterrupted trial enrollment and treatment delivery across diverse care settings
Integrative segmentation revealing clinical, logistical, and commercial differentiators across therapy types, administration routes, patient lines, biomarkers, settings, and distribution channels
Meaningful segmentation illuminates where clinical benefit, commercial opportunity, and operational complexity converge, and it requires attentive cross-mapping of therapeutic modality, route of administration, care setting, biomarker profile, line of therapy, and distribution pathways. When viewed by treatment type, stakeholders must reconcile the differing clinical and logistical demands of chemotherapy-distinguishing between platinum-based regimens and non-platinum agents-with the targeted precision of anti-angiogenic therapies and PARP inhibitors, the systemic immunomodulation pursued through checkpoint inhibitors and cancer vaccines, and the definitive role of surgery in selected recurrent presentations. Administration route further differentiates patient experience and resource burden; intraperitoneal therapies pose distinct procedural requirements compared with intravenous infusions, while oral and subcutaneous modalities support outpatient and home-based care models that reduce facility dependency.Line of therapy segmentation clarifies where therapeutic intensity and evidence thresholds evolve, distinguishing the needs of patients receiving second-line, third-line, and fourth-line and beyond treatments and highlighting where safety, tolerability, and quality-of-life become dominant decision drivers. Biomarker stratification-BRCA mutation status and broader homologous recombination deficiency classification-remains central to treatment selection and response probability, reinforcing the clinical imperative for accessible, reliable diagnostic testing. End-user distinctions underscore how ambulatory care centers, cancer centers, hospitals, and specialty clinics differ in procedural capabilities, infusion capacity, and payer contracting, which in turn affects therapy uptake and care continuity. Finally, distribution channel segmentation-hospital pharmacies, online pharmacies, and retail pharmacies-shapes reimbursement workflows, patient access points, and inventory logistics, particularly for oral agents and complex supportive therapies. Integrating these segments into program design enables more precise go-to-market planning and operational execution across the care continuum
Regional access and infrastructure dynamics that determine adoption pathways and evidence priorities across the Americas, Europe Middle East Africa, and Asia Pacific
Regional dynamics reflect a mix of demographic demand, regulatory nuance, diagnostic infrastructure, and reimbursement paradigms that together shape therapy adoption and access. In the Americas, strong diagnostic capabilities, widespread adoption of targeted therapies, and large integrated health systems support rapid translation of biomarker-driven approaches, but heterogeneity in payer coverage and pricing pressure requires nuanced access strategies and value evidence tailored to private and public payers. Europe, the Middle East & Africa presents a diverse regulatory and reimbursement mosaic; certain European markets provide robust pathway support for precision diagnostics and innovative agents, whereas other jurisdictions and many countries in the Middle East & Africa face constrained diagnostic capacity and budgetary limits that necessitate tiered access approaches and strengthening of local diagnostic networks.Asia-Pacific encompasses rapidly evolving oncology ecosystems with significant heterogeneity in regulatory timelines, domestic manufacturing capacity, and reimbursement frameworks. Several jurisdictions are expanding genomic testing infrastructure and accelerating approvals for targeted agents, while others continue to prioritize cost-containment and local production. Across regions, cross-border collaboration on clinical trials, enhanced local regulatory engagement, and investments in diagnostic capacity can accelerate equitable access to contemporary therapeutic options. A regionally differentiated approach that aligns evidence generation, pricing strategies, and supply chain plans with local health system realities will be essential for sustained impact
Competitive corporate strategies and cross-sector partnerships that accelerate biomarker integration, supply chain resilience, and payer evidence generation in oncology
Corporate strategies among leading oncology developers and specialty pharmaceutical firms are increasingly characterized by focused portfolios, strategic alliances, and diversified capabilities across small molecules, biologics, and companion diagnostics. Key organizations are prioritizing biomarker-driven indications and lifecycle management for maintenance therapies, while others are expanding into novel modalities such as antibody-drug conjugates and cellular approaches. Partnerships with diagnostic providers have become a commercial imperative to ensure appropriate patient identification and to support label-concordant use of targeted agents. Moreover, cross-sector collaborations-linking biopharma with contract manufacturers, logistics specialists, and digital health vendors-are enabling more resilient supply chains and patient-centric service models.Competitive dynamics also reflect a maturation in real-world evidence generation and health economic modeling, where firms invest in longitudinal data assets and payer-facing dossiers that demonstrate comparative effectiveness and value. This focus on evidence and partnership-driven commercialization indicates that future differentiation will depend as much on diagnostic integration, manufacturing agility, and channel excellence as on molecular innovation alone. Companies that align clinical development with pragmatic deployment strategies and that secure strategic partnerships across the ecosystem will be better positioned to implement therapies at scale and to respond rapidly to evolving clinical guidance
Practical, high-impact actions for drug developers and service providers to align diagnostics, evidence, supply resilience, and access to optimize therapy adoption
Industry leaders must pursue coordinated actions that bridge clinical evidence, diagnostic access, and operational readiness to ensure new therapies translate into meaningful patient benefit. Begin by prioritizing early and systematic biomarker strategies that align diagnostic development with therapeutic trials, ensuring companion assays are validated, reimbursable, and available across key care settings. Simultaneously, design clinical programs that emphasize tolerability and patient-reported outcomes for later-line populations, and pursue combination strategies only where there is a clear mechanistic rationale supported by translational biomarkers and safety data.Operationally, invest in supply chain diversification and flexible manufacturing arrangements to mitigate tariff-driven and geopolitical disruptions, and establish robust distribution contingencies spanning hospital, retail, and online channels. Engage payers and health technology assessment bodies early, offering real-world evidence generation plans and value-based contracting options to ease formulary access. Finally, foster partnerships with centers of excellence and community providers to build diagnostic capacity and care pathways that support timely treatment initiation, adherence, and longitudinal monitoring. These actions, taken together, will reduce commercial friction, protect trial continuity, and accelerate patient access to clinically appropriate therapies
Transparent, evidence-integrative methodology combining clinical trials, guideline updates, and real-world evidence to inform practical strategic insights and identify evidence gaps
This research synthesizes multiple evidence streams to provide a rigorous, transparent foundation for analysis. Primary inputs include peer-reviewed clinical trials, guideline updates, regulatory approvals, and regulatory committee deliberations, complemented by published real-world studies that illuminate utilization patterns, safety, and outcomes across diverse care settings. Secondary inputs draw on public filings, company disclosures, conference proceedings, and technical guidance documents for diagnostics and therapeutic modalities. Wherever possible, analytic claims are supported by cross-referencing pivotal randomized data with observational datasets to capture both efficacy and pragmatic implementation considerations.Analytical methods emphasize qualitative synthesis, comparative evidence mapping, and scenario-based assessment to identify drivers of clinical and commercial relevance. Segmentation frameworks were constructed to parallel clinical decision points-treatment modality, administration route, line of therapy, biomarker profile, care setting, and distribution channel-enabling targeted insight generation. Limitations are acknowledged and include variability in HRD assay harmonization, heterogeneity in real-world data capture across regions, and ongoing evolution of combination therapy evidence. The methodology therefore prioritizes transparency and replicability while highlighting areas where targeted primary research or prospective evidence generation could refine conclusions
Synthesis of clinical, diagnostic, and operational imperatives showing how integrated strategies can convert innovation into accessible therapeutic impact for patients
Advanced recurrent ovarian cancer now sits at an inflection point in which precision diagnostics, diversified therapeutic modalities, and evolving delivery models converge to create new opportunities and real-world complexities. The field benefits from targeted agents that have redefined maintenance and late-line strategies for biomarker-selected patients, while contemporaneous advances in diagnostics and care pathways enable earlier and more accurate treatment alignment. At the same time, immunotherapy and novel combination approaches demand thoughtful translational strategies and rigorous patient selection to realize their potential. Operational challenges-ranging from supply chain fragility to variable regional diagnostic capacity-underscore the need for pragmatic, regionally tailored plans that connect clinical innovation to sustainable access.In conclusion, stakeholders who integrate biomarker-driven development, evidence generation centered on patient-reported and comparative outcomes, and resilient commercial operations will be best positioned to deliver therapeutic advances to patients with advanced recurrent ovarian cancer. The path forward requires interdisciplinary collaboration, targeted investments in diagnostics and distribution, and proactive engagement with payers and providers to translate scientific progress into measurable clinical impact.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Advanced Recurrent Ovarian Cancer Market
Companies Mentioned
The key companies profiled in this Advanced Recurrent Ovarian Cancer market report include:- AbbVie Inc.
- Amgen Inc.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Celsion Corporation
- Clovis Oncology Inc.
- Debiopharm International SA
- Eli Lilly and Company
- Exelixis Inc.
- F. Hoffmann-La Roche Ltd.
- Genentech Inc.
- GlaxoSmithKline plc
- ImmunoGen Inc.
- Johnson & Johnson Services, Inc.
- Kintara Therapeutics Inc.
- Merck & Co., Inc.
- Mirati Therapeutics Inc.
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Vivesto AB
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
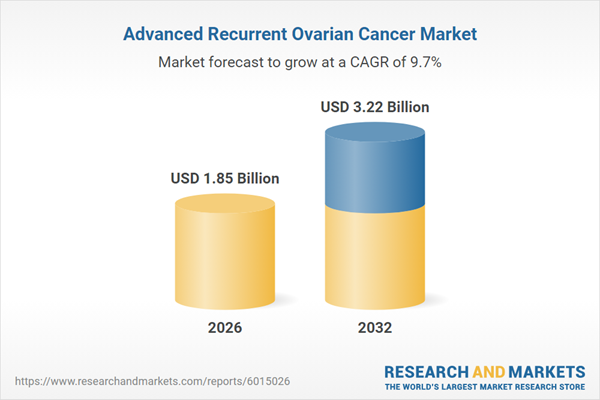
| Estimated Market Value ( USD | $ 1.85 Billion |
| Forecasted Market Value ( USD | $ 3.22 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |