Speak directly to the analyst to clarify any post sales queries you may have.
A concise orientation explaining how molecular precision, modality diversity, and cross‑functional strategy are redefining development and commercialization of targeted therapies
Targeted therapy sits at the intersection of molecular science, precision diagnostics, and patient-centric care, reshaping how oncologic and immunologic diseases are treated. Recent advances in molecular targeting, conjugation chemistry, and targeted protein degradation have expanded the palette of therapeutic modalities, enabling medicines that reach specific cellular vulnerabilities while reducing systemic toxicity. As clinical practice evolves to emphasize biomarker-driven treatment selection, stakeholders across industry and health systems face both opportunity and complexity in bringing next-generation targeted therapies to patients.Translational pipelines are now characterized by greater modality diversity than a decade ago, with engineered antibodies, antibody drug conjugates, small molecule inhibitors, and emerging protein degraders each offering distinct operational, regulatory, and commercial profiles. This diversification requires integrated strategies across discovery, manufacturing, regulatory affairs, clinical development, and commercial operations. Consequently, strategic leaders must balance deep scientific specialization with scalable manufacturing and pragmatic payer engagement to ensure therapies achieve both approval and adoption.
The following analysis synthesizes pivotal shifts in the landscape, regulatory and trade headwinds, segmentation insights, and regional dynamics that will inform near-term and medium-term planning. It is written to support executives and strategic teams as they prioritize investments, design partnerships, and prepare operational capabilities that align with the demands of a rapidly advancing therapeutic ecosystem.
A forward‑looking synthesis of scientific advances, regulatory evolution, manufacturing modernization, and commercial shifts that are redefining targeted therapy strategy
The targeted therapy landscape is undergoing transformative shifts driven by scientific innovation, changing regulatory expectations, and evolving reimbursement practices. Advances in molecular biology and platform technologies have enabled a surge of modular approaches: next‑generation antibody constructs extend specificity and payload delivery, while protein degradation platforms present an orthogonal mechanism to inhibit previously undruggable targets. These scientific shifts are not isolated; they cascade into clinical trial design, biomarker development, and companion diagnostics, requiring closer alignment between translational research teams and clinical operations.Concurrently, regulatory bodies are adopting more adaptive pathways and heightened emphasis on real‑world evidence, compounding the need for robust post‑market data strategies across stakeholders. Payer models are pivoting toward outcomes‑based agreements and indication‑specific contracting, incentivizing manufacturers to demonstrate value across heterogeneous patient populations. As a result, commercial strategies are evolving to prioritize early health economics planning and cross‑discipline evidence generation.
Manufacturing and supply chain innovations are also reshaping feasibility and cost structures. Single‑use technologies, modular facilities, and advances in analytical characterization reduce lead times for complex biologics while enabling flexible capacity allocation across modalities. Digital health tools and decentralized trial models accelerate patient recruitment and enable richer longitudinal data collection, improving the speed and robustness of evidence generation. Finally, strategic partnerships-linking small biotech innovators with specialized contract development and manufacturing organizations-are migrating from transactional relationships to integrated, long‑term collaborations that share risk and align incentives, thus accelerating translation from bench to bedside.
An evidence‑based examination of how recent US tariff measures in 2025 are reshaping supply chains, manufacturing decisions, trade compliance, and payer negotiations across targeted therapy value chains
The implementation of new tariff measures in the United States during 2025 has introduced layered implications for the targeted therapy sector that extend beyond direct input pricing. Tariffs on raw materials and certain biologics‑related reagents can increase the cost base for active pharmaceutical ingredient production and analytical consumables, exerting pressure on manufacturers who operate just‑in‑time supply models. In turn, manufacturers are evaluating procurement diversification and increased domestic sourcing where feasible, while also reassessing inventory policies to mitigate short‑term disruption risk.Downstream effects include shifts in contract negotiations with CDMOs and logistics providers, as entities seek to realign commercial terms to reflect higher landed costs and uncertain cross‑border flows. For organizations heavily reliant on global supply chains, tariffs can precipitate strategic decisions to regionalize manufacturing footprints or to invest in redundant supplier relationships to ensure continuity of production. These responses carry implications for capital allocation, timeline fidelity for clinical supply, and long‑term unit economics.
From a commercial and access perspective, increased cost pressure can influence pricing discussions with payers and formulary committees, especially in markets where value frameworks are sensitive to therapy acquisition costs. Manufacturers may prioritize indication sequencing, focusing on high‑value, high‑unmet‑need indications that offer clearer value propositions. Simultaneously, companies might intensify efforts to demonstrate downstream savings through reduced hospitalizations or improved patient outcomes to preserve reimbursement leverage.
Regulatory and compliance pathways are also affected as import and export documentation, customs processes, and quality release testing become more prominent in operational planning. Companies must ensure that changes to supplier networks do not compromise regulatory commitments or the integrity of product dossiers. In response, legal and regulatory teams are playing a more central role in commercial strategy, working to harmonize trade compliance with clinical supply continuity and market access timelines.
Finally, the tariff landscape has catalyzed collaborative problem solving across the ecosystem. Industry consortia, supplier coalitions, and strategic alliances are emerging to pool resources, advocate for targeted exemptions where appropriate, and develop shared mitigation frameworks. These cooperative approaches aim to protect patient access and preserve the momentum of therapeutic innovation despite macroeconomic headwinds.
A granular exploration of product modalities, administration pathways, and end‑user channels revealing modality‑specific operational and commercial imperatives for targeted therapies
Understanding the market through segmentation reveals nuanced operational and strategic considerations across product classes, administration routes, and end‑user channels. Product heterogeneity is pronounced: antibody drug conjugates include CD22‑directed constructs and HER2‑directed constructs, each with unique conjugation chemistries, linker stability considerations, and payload selection challenges; monoclonal antibodies present as chimeric, fully human, or humanized formats that differ in immunogenicity risk, manufacturing complexity, and biosimilar competitive dynamics; protein degraders encompass molecular glues and PROTACs, both introducing distinct medicinal chemistry and safety monitoring paradigms; small molecule inhibitors span PARP inhibitors, proteasome inhibitors, and tyrosine kinase inhibitors, which vary in oral bioavailability, drug-drug interaction profiles, and chronic administration considerations.Route of administration further differentiates clinical and commercial strategies. Intravenous therapies typically demand hospital or infusion center infrastructure, impacting distribution logistics, cold chain management, and site contracting. Oral administration shifts focus to adherence strategies, patient education, and outpatient monitoring, while subcutaneous delivery introduces opportunities for at‑home administration and reduced facility burden, but requires attention to device design and patient support programs.
End‑user segmentation shapes adoption pathways and service models. Home care settings and specialty clinics can accelerate patient convenience and adherence when supported by robust training and nursing programs, whereas hospital pharmacies and retail pharmacies play critical roles in formulary placement, inventory management, and point‑of‑care logistics. Each channel imposes different requirements for cold‑chain oversight, patient counseling resources, and reimbursement interaction, mandating tailored commercial and operational playbooks.
Taken together, these segmentation lenses highlight that successful product launches and lifecycle management hinge on integrated planning across modality science, delivery format, and channel engagement. Cross‑functional alignment-linking development teams, commercial leaders, and supply chain planners-will be essential to translate modality‑specific attributes into differentiated patient and payer propositions.
A comparative regional analysis detailing how regulatory, reimbursement, and manufacturing dynamics differ across the Americas, Europe Middle East & Africa, and Asia‑Pacific regions and what that means for strategic planning
Regional dynamics exert a powerful influence on development priorities, commercialization tactics, and supply chain design across the targeted therapy ecosystem. In the Americas, regulatory pathways and payer environments often demand robust comparative effectiveness evidence and detailed pharmacoeconomic rationales, which shape development sequencing and post‑launch evidence generation. The sheer scale and heterogeneity of healthcare delivery systems across the region also encourage modular go‑to‑market strategies that combine national payer engagements with localized provider education and access programs.In Europe, Middle East & Africa, market access negotiations frequently involve health technology assessment scrutiny and country‑level reimbursement determinations that can vary substantially in timelines and evidentiary expectations. This region’s diverse regulatory frameworks and procurement models necessitate flexible launch strategies, including managed entry agreements and differentiated pricing architectures designed to align value demonstration with local needs.
In Asia‑Pacific, the policy focus often combines rapid adoption of novel therapies in certain markets with strong domestic manufacturing ambitions and growing investment in biosimilars and biomanufacturing infrastructure. Strategic partnerships with local clinical networks, investments in regional manufacturing capacity, and culturally attuned patient support initiatives are central to successful market entry and scale in this region. Across all regions, geopolitical and trade factors influence supply chain choices and inventory planning, reinforcing the need for geographically diversified risk management and regulatory harmonization efforts.
A synthesis of prevailing corporate archetypes, partnership models, and operational plays that clarify how different types of companies are positioning to win in the evolving targeted therapy ecosystem
Company strategies in the targeted therapy domain fall into a set of discernible archetypes that reflect differing risk appetites, asset portfolios, and go‑to‑market capabilities. Large integrated pharmaceutical companies typically leverage scale to support late‑stage development, global commercialization, and complex manufacturing, while focusing internal R&D on platform technologies that can be applied across therapeutic areas. These organizations often pursue strategic partnerships, licensing deals, and selective acquisitions to complement internal pipelines and to access niche modality expertise.Biotechnology innovators tend to concentrate on high‑value science and early proof‑of‑concept, aiming to de‑risk programs through focused clinical studies and then seek collaboration with larger partners for global scale. Contract development and manufacturing organizations are increasingly strategic partners rather than vendors, investing in platform technologies, analytical capabilities, and capacity expansions that can accommodate complex biologics and conjugated molecules.
Diagnostic and precision medicine firms play a pivotal role in enabling patient selection and treatment optimization, driving closer integration between companion diagnostics and therapeutic development. Meanwhile, specialty distributors and service providers are developing integrated solutions that combine logistics, patient support, and reimbursement navigation to address channel‑specific challenges. Across these archetypes, successful companies emphasize cross‑functional integration, early health economics planning, and iterative evidence generation to sustain adoption and to navigate complex regulatory and payer environments.
A pragmatic set of prioritized actions and cross‑functional initiatives designed to accelerate adoption, de‑risk supply chains, and strengthen value realization for targeted therapies
Industry leaders should adopt a set of prioritized actions to navigate scientific opportunity while mitigating operational and commercial risk. First, align modality selection with clear payer and clinical value pathways early in development by integrating health economics and outcomes research into phase II and III design. This alignment reduces downstream negotiation friction and strengthens formulary positioning. Second, diversify supply chains and invest in regional manufacturing or dual‑sourcing strategies to mitigate trade‑related disruptions and to preserve clinical supply continuity during geopolitical shifts.Third, pursue modular partnerships with specialized service providers and diagnostic firms to accelerate patient identification and improve time‑to‑treatment; these collaborations should be structured with shared milestones and data‑sharing agreements to ensure alignment. Fourth, design patient‑centric access mechanisms tailored to administration routes, investing in adherence support for oral agents and scalable home‑administration programs for subcutaneous formulations. These programs should be measurable and linked to real‑world outcome collection to inform payer discussions.
Fifth, integrate digital analytics and real‑world evidence platforms to support adaptive regulatory pathways and post‑market commitments, enabling rapid iteration of safety and effectiveness profiles. Sixth, prioritize talent and organizational structures that favor cross‑functional fluency, ensuring R&D, regulatory, supply chain, and commercial teams collaborate from the earliest program stages. Taken together, these actions will strengthen resilience, enhance adoption, and optimize the translation of scientific innovations into sustainable patient benefit.
A transparent explanation of the mixed‑methods research approach combining secondary literature synthesis, expert qualitative interviews, scenario analysis, and methodological safeguards to ensure robust insights
The research methodology underpinning this analysis combined a structured review of scientific literature, regulatory guidance, and industry reports with primary qualitative interviews and internal cross‑validation exercises. Secondary research focused on peer‑reviewed publications and regulatory filings to characterize modality attributes, clinical outcomes, and safety profiles. These sources informed thematic synthesis on innovation trends and evidence generation strategies.Primary research involved in‑depth interviews with clinical investigators, regulatory experts, supply chain leaders, and commercial strategists to capture operational implications and real‑world perspectives. Interview insights were triangulated with public regulatory announcements and product labeling to ensure consistent interpretation. Analytical methods included scenario mapping to explore tariff‑driven supply chain impacts, and segmentation modeling to align modality characteristics with route of administration and end‑user considerations.
Quality assurance steps included cross‑verification of technical claims with subject matter experts and sensitivity checks for trade‑related impact assessments. Where uncertainty remained, qualitative caveats were documented, and recommendations were framed to accommodate variability in regional policy and market conditions. The methodology emphasizes transparency, reproducibility of analytic logic, and practical relevance for decision makers seeking to translate findings into strategic action.
A closing synthesis emphasizing the imperative for integrated scientific, operational, and commercial strategies to translate targeted therapy innovation into durable patient and business outcomes
Targeted therapies represent a rapidly maturing segment of modern therapeutics, driven by modality diversification, precision medicine, and evolving commercial models. Scientific advances provide multiple avenues to target disease biology more precisely, yet these innovations introduce operational complexity that spans manufacturing, regulatory strategy, and payer engagement. External factors such as trade policies and regional regulatory heterogeneity further complicate decision making, underscoring the need for integrated, resilient strategies.To succeed, stakeholders must blend deep technical expertise with nimble commercial and operational planning. That means investing in diversified supply chains, establishing pragmatic partnerships, designing evidence generation that addresses payer expectations, and developing patient‑centric delivery models that reflect real‑world usage. Organizations that align scientific ambition with practical deployment capabilities will be best positioned to translate breakthroughs into sustained patient impact and commercial success.
This analysis aims to provide strategic clarity by mapping the principal drivers of change, the operational implications of policy shifts, and the segmentation and regional considerations that inform tactical choices. It is intended to serve as a foundation for leadership teams as they prioritize investments, structure partnerships, and prepare for the next wave of targeted therapy innovation.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
15. China Targeted Therapy Market
Companies Mentioned
The key companies profiled in this Targeted Therapy market report include:- AbbVie Inc.
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Bayer AG
- BeiGene, Ltd.
- Biogen Inc.
- BioMarin Pharmaceutical Inc.
- Bristol Myers Squibb Company
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- F. Hoffmann-La Roche AG
- Genmab A/S
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Incyte Corporation
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Merck KGaA
- Novartis AG
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
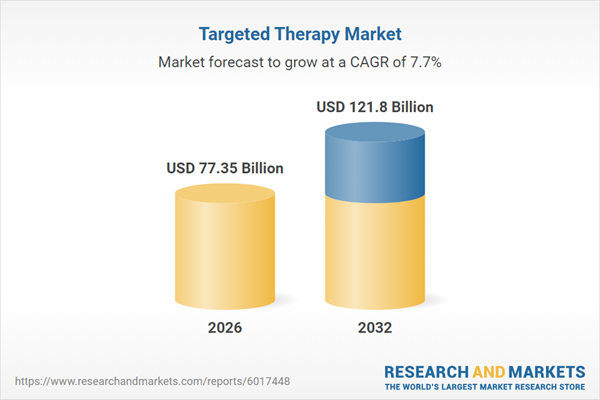
| Estimated Market Value ( USD | $ 77.35 Billion |
| Forecasted Market Value ( USD | $ 121.8 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |