Speak directly to the analyst to clarify any post sales queries you may have.
A forward-looking orientation to fluorescence in situ hybridization probes that frames technological maturity, clinical adoption, and translational value across research and diagnostics
Fluorescence in situ hybridization probes have evolved from specialized research tools into indispensable clinical and translational instruments that bridge genomic insight with diagnostic action. Advances in probe chemistry, imaging modalities, and automation have collectively improved analytical sensitivity and workflow compatibility, enabling broader integration within pathology laboratories and research centers alike. These developments have accelerated the translation of cytogenetic and molecular findings into clinically actionable diagnoses, particularly where single-cell resolution and spatial context are required.The current landscape is characterized by intensified cross-disciplinary collaboration among molecular biologists, clinical cytogeneticists, and diagnostic innovators. As laboratories seek to balance throughput with the need for precise localization of nucleic acid targets, FISH probes have become integral to diagnostic algorithms for cancer and genetic disorders, while also gaining traction in infectious disease and prenatal contexts. This introduction frames the report’s subsequent analysis by emphasizing technological maturity, regulatory considerations, and the growing demand for robust, reproducible assays that can operate across diverse end users from academic research to clinical laboratories.
Fundamental shifts in probe chemistry, multiplexing, and integration with digital pathology that are redefining spatial genomic analysis and clinical workflows
The landscape of fluorescence in situ hybridization probes is witnessing transformative shifts driven by innovations in probe design, multiplexing capability, and integration with digital pathology. Improvements in fluorophore chemistry and signal amplification methods have expanded the range of detectable targets while reducing background noise, thereby enabling more confident interpretation of complex samples. Concurrently, multiplexing strategies are allowing simultaneous interrogation of multiple loci, which is reshaping diagnostic workflows by reducing time-to-result and conserving precious tissue samples.Another pivotal shift is the convergence of FISH with complementary technologies such as next-generation sequencing and single-cell analytics. This hybridization of methodologies is redefining biomarker discovery and validation pathways, permitting orthogonal confirmation of genomic events and enabling more nuanced patient stratification. In parallel, increasing automation and standardization are lowering technical barriers for routine clinical adoption, making FISH assays more accessible to diagnostic laboratories and hospitals. Regulatory attention and quality assurance frameworks are also maturing, prompting manufacturers and laboratory developers to prioritize assay reproducibility, traceability, and user-centric design. Taken together, these shifts are not merely incremental improvements but represent a recalibration of how spatial genomics informs both research priorities and clinical decision-making.
Strategic operational responses and procurement adaptations in reaction to recent tariff pressures affecting supply chains and sourcing strategies for diagnostic probes
Recent tariff developments in the United States have introduced an added dimension of operational complexity for suppliers and purchasers of FISH probes and related consumables. Supply chain managers and procurement teams are reassessing sourcing strategies and inventory policies to mitigate potential cost volatility. This reappraisal has accelerated efforts to diversify supplier bases and to evaluate local manufacturing or near-shore partnerships where feasible, with the objective of preserving continuity of clinical and research services.Laboratories and institutional buyers are responding by examining vendor agreements and prioritizing long-term supply assurances over short-term price gains. Concurrently, developers of probes and kits are considering greater vertical integration or contractual manufacturing relationships to shield critical components from cross-border tariff exposure. While these measures introduce new operational considerations, they also incentivize greater transparency in component origin and production processes. As a result, decision-makers are increasingly weighing total cost of ownership, lead-time reliability, and regulatory compliance together when selecting FISH probe solutions, rather than focusing solely on unit price.
Comprehensive segmentation-driven insights that map application requirements, end-user behaviors, probe types, and label chemistries to product and commercialization strategies
A nuanced understanding of product and end-user segmentation is essential for designing targeted development and commercialization strategies. When examining applications, it is important to distinguish between Cancer Diagnosis, Genetic Disorder Diagnosis, Infectious Disease Diagnosis, and Prenatal Diagnosis; within cancer, attention must be given to hematologic malignancies versus solid tumors, and within genetic disorder testing the diagnostic approaches for chromosomal abnormalities differ from those for single-gene mutations. Each application imposes distinct analytical requirements on probe design, labeling chemistry, and sensitivity thresholds, and thus drives differentiated product specifications and validation pathways.End-user segmentation further informs go-to-market and support models. Academic and research institutes operate with different procurement rhythms and validation expectations than biopharmaceutical companies, diagnostic laboratories, or hospitals and clinics; moreover, academic buyers can be categorized into government research organizations and private research organizations, each with their own funding cycles and collaboration patterns. Probe-type differentiation between direct labeled probes and indirect labeled probes has implications for assay complexity, signal intensity, and workflow steps, influencing buyer preferences based on laboratory capability and throughput expectations. Finally, label type-whether fluorescent-labeled probes or hapten-labeled probes-affects detection modality, compatibility with instrumentation, and long-term archival stability of slides. Integrating these segmentation dimensions provides a layered view of product-market fit that supports tailored product development, regulatory planning, and customer engagement strategies.
Regionally differentiated dynamics and strategic considerations for building adoption pathways across the Americas, Europe Middle East & Africa, and Asia-Pacific markets
Regional dynamics play a determinative role in how FISH probe technologies are developed, regulated, and adopted. The Americas exhibit a strong concentration of translational research activity and advanced clinical laboratories, supported by a robust ecosystem of diagnostic manufacturers and contract research organizations. Regulatory ecosystems and reimbursement pathways influence adoption timelines, while academic and hospital networks act as early adopters for innovative assays.Europe, Middle East & Africa present a heterogeneous environment where regulatory frameworks, healthcare infrastructure maturity, and public health priorities can vary significantly across subregions. This variability necessitates differentiated market entry and evidence generation strategies, with emphasis on local clinical validation and stakeholder engagement. Asia-Pacific is characterized by rapid clinical expansion, growing investment in molecular diagnostics, and increasing domestic manufacturing capabilities. The region’s diverse health systems and large patient populations offer substantial opportunities for scale, but also require attention to localization of assay workflows, training, and supply continuity. Understanding these regional nuances enables more effective allocation of commercial resources and strategic partnerships tailored to local market realities.
Competitive profiles and partnership dynamics that favor integrated technical expertise, scalable manufacturing, and evidence-driven adoption strategies in probe development
Competitive dynamics in the FISH probe arena are shaped by a mix of established reagent manufacturers, specialized probe developers, instrument vendors offering integrated solutions, and academic spinouts translating niche technologies. Leaders with deep expertise in probe chemistry and manufacturing scalability distinguish themselves by offering validated kits, robust technical support, and regulatory documentation that streamlines laboratory accreditation processes. At the same time, smaller, innovation-focused companies often introduce disruptive approaches such as novel labeling methods or highly multiplexed probe sets that can alter competitive positioning if paired with credible clinical evidence and commercial partnerships.Collaboration between diagnostic companies and clinical laboratories or research institutions is increasingly important for evidence generation and iterative product improvement. Strategic alliances that combine manufacturing scale with technical innovation accelerate time-to-adoption and mitigate the risks associated with new assay introduction. In addition, providers that invest in comprehensive training programs, digital tools for interpretation, and workflow integration services create higher switching costs for customers and foster long-term relationships that extend beyond single kit sales. The combined effect of these dynamics underscores the need for companies to balance R&D intensity with operational excellence in manufacturing, regulatory navigation, and customer enablement.
Actionable strategic priorities for manufacturers and service providers to translate probe innovation into reliable, scalable, and clinically adopted diagnostic solutions
Industry leaders should prioritize investments that align technical innovation with pragmatic deployment needs. First, accelerating work on multiplexed probe sets and improved fluorophores should be paired with usability-focused design that reduces hands-on time and minimizes interpretative variability. This dual emphasis on capability and operational simplicity will facilitate broader uptake across hospital laboratories and diagnostic centers. Second, manufacturers and distributors should strengthen supply-chain resilience by diversifying component sourcing, evaluating localized production options, and establishing contingency inventory plans to limit disruption from trade policy shifts.Additionally, organizations should expand collaborations with clinical networks and research consortia to generate pragmatic evidence that resonates with laboratory directors and payers. Investing in standardized training modules and digital interpretation aids will reduce barriers to adoption and increase the perceived value of higher-priced, higher-performance assays. Finally, companies must maintain proactive regulatory engagement and quality frameworks that anticipate evolving accreditation requirements. By aligning R&D priorities, manufacturing reliability, and customer support, industry leaders can translate technological advances into sustainable clinical and commercial outcomes.
A rigorous mixed-methods research framework combining stakeholder interviews, literature synthesis, and supply-chain analysis to ensure evidence-based insights and practical recommendations
The research approach underpinning this report synthesizes primary and secondary methods to ensure robust triangulation of findings. Primary research included structured discussions with clinical laboratory directors, procurement specialists, assay developers, and academic investigators to capture practical considerations around assay performance, workflow constraints, and validation priorities. These conversations provided contextual insight into adoption drivers and operational trade-offs that quantitative datasets may not fully convey.Secondary research entailed systematic review of scientific literature, regulatory guidance documents, patent filings, and publicly available clinical trial registries to map technological advancements and evidence generation trends. Supply-chain analysis incorporated trade flow data and supplier disclosures to identify vulnerabilities and diversification opportunities. Where applicable, case examples and technology deep-dives were employed to illustrate best-practice implementation and to delineate how specific probe chemistries translate into laboratory outcomes. Cross-validation between primary insights and secondary sources ensured that recommendations are grounded in observable practice and emerging scientific consensus.
Concluding synthesis on how technological improvements, operational resilience, and evidence generation will together determine sustained clinical and research adoption of FISH probes
The collective findings emphasize that fluorescence in situ hybridization probes occupy a pivotal role at the intersection of molecular precision and spatial context, offering unique diagnostic and research value that complements other genomic technologies. Key drivers of future progress will be the maturation of multiplexing approaches, continued improvements in labeling chemistries, and the integration of digital interpretation tools that enhance reproducibility and throughput. These advances will enable more nuanced clinical decision-making, particularly in oncology and genetic disorder diagnosis, while also supporting expanded use in prenatal and infectious disease settings.Adoption will hinge on a balanced approach that couples technological sophistication with pragmatic considerations such as ease of use, supply stability, and demonstrable clinical utility. Organizations that invest in evidence generation, customer enablement, and resilient operations will be best positioned to capture opportunities as laboratories and health systems increasingly seek assays that deliver reliable spatial genomic information within routine workflows. The conclusion underscores the need for coordinated efforts across R&D, manufacturing, regulatory, and commercialization functions to convert scientific promise into sustained clinical impact.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Fluorescence In Situ Hybridization Probe Market
Companies Mentioned
The key companies profiled in this Fluorescence In Situ Hybridization Probe market report include:- Abbott Laboratories
- Abnova Corporation
- Agilent Technologies Inc
- Bio-Rad Laboratories Inc
- Bio-Techne
- BioCare Medical LLC
- BioDot
- BioGenex Laboratories Inc
- BioView
- Creative Biolabs
- Cytocell Ltd
- CytoTest Inc
- Danaher Corporation
- Empire Genomics LLC
- Euroclone SpA
- F. Hoffmann-La Roche Ltd
- Genemed Biotechnologies Inc
- Horizon Diagnostics
- Leica Biosystems Nussloch GmbH
- LGC Biosearch Technologies
- MetaSystems Probes GmbH
- Oxford Gene Technology IP Limited
- PerkinElmer Inc
- QIAGEN N.V.
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
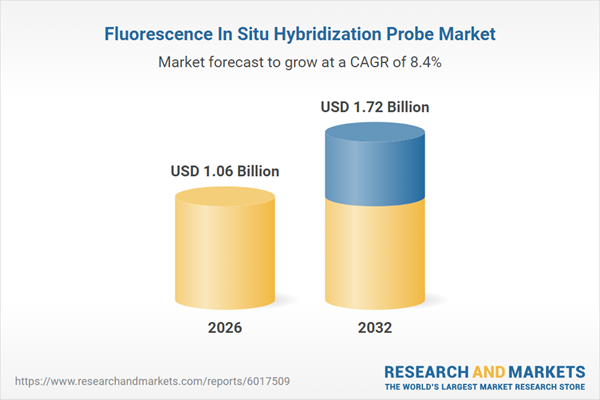
| Estimated Market Value ( USD | $ 1.06 Billion |
| Forecasted Market Value ( USD | $ 1.72 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |