Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Substantial financial commitment to scientific innovation further underpins this sector, directly fueling the usage of culture vessels. As reported by EFPIA, the research-based pharmaceutical industry in Europe allocated an estimated €55.00 billion toward R&D in 2024. However, despite these favorable trends, the market confronts a significant obstacle regarding contamination control; the persistent threat of cross-contamination during culture processes poses a risk of expensive batch failures and compromised experimental data, representing a critical challenge for both manufacturers and researchers.
Market Drivers
The escalating global demand for vaccines and biopharmaceuticals acts as a primary catalyst for the cell culture vessels market. As pharmaceutical developers focus on producing monoclonal antibodies and viral vectors, there is an intensified need for reliable, sterile containment systems like roller bottles and multi-well plates to facilitate large-scale upstream processing. This manufacturing momentum is highlighted by significant capital allocation toward infrastructure; for example, Novartis announced in an April 2025 corporate press release a plan to invest $23 billion over five years to broaden its U.S. manufacturing and R&D footprint. Such expansion translates into financial gains for consumable suppliers, with Corning Incorporated reporting in their January 2025 financial results that Life Sciences sales rose 8% to $2.0 billion for the year, largely due to sustained bioprocessing demand.The rapid expansion of the stem cell and regenerative medicine industries further accelerates market growth by necessitating specialized cultivation environments. Unlike traditional biologics, cell and gene therapies often require advanced vessel geometries to ensure optimal gas exchange and cell adherence during the cultivation of autologous or allogeneic batches. This sector continues to attract significant funding, ensuring a steady procurement channel for high-performance culture apparatus. According to the Alliance for Regenerative Medicine's presentation at the 'Cell & Gene Meeting on the Mesa' in October 2024, global sector investments reached $10.9 billion in the first half of 2024 alone, funding clinical advancements that directly translate into increased volume requirements for critical single-use bags and flasks.
Market Challenges
Contamination control remains a formidable impediment to the Global Cell Culture Vessels Market, significantly constraining financial performance and operational efficiency. The inherent risk of cross-contamination during sensitive biopharmaceutical production forces manufacturers to implement extremely rigorous validation and sterilization measures. These necessary but burdensome protocols increase operational costs and extend production lead times, thereby reducing overall throughput. Furthermore, actual contamination events result in catastrophic batch failures, necessitating the disposal of high-value biologic products and causing severe delays in bringing critical therapies to market.The industry's intense focus on mitigating these risks is quantitatively evident. According to the Parenteral Drug Association (PDA) in 2024, over 80% of surveyed manufacturers upgraded their validation protocols to meet tightening contamination control standards. This substantial allocation of resources toward sterility assurance and compliance diverts capital from innovation and capacity expansion. Consequently, the persistent threat of contamination not only jeopardizes product integrity but also creates high barriers to scalability and market entry, directly hampering the sector's growth trajectory.
Market Trends
The development of automation-compatible vessel designs is accelerating as biopharmaceutical manufacturers prioritize operational consistency and high-throughput screening. To integrate seamlessly with robotic plate movers and liquid handlers, vessel engineering is shifting toward standardized microplate footprints featuring rigid, gripper-friendly structures that eliminate the variability associated with manual manipulation. This industrial push for standardized, automation-ready consumables is reflected in the financial performance of major suppliers; for instance, Sartorius Stedim Biotech reported in its January 2025 preliminary results for fiscal 2024 that it generated sales revenue of €2.78 billion, noting a distinct revitalization in its consumables business as customers normalized inventory levels to support automated bioprocessing activities.Simultaneously, the emergence of specialized vessels for three-dimensional cell culture is transforming drug discovery workflows by providing environments that are more physiologically relevant than traditional monolayers. Researchers are increasingly procuring advanced scaffolds, microfluidic chips, and ultra-low attachment plates designed to facilitate the growth of organoids and spheroids, which offer superior predictive data for efficacy and toxicity testing. This demand for sophisticated culture platforms contributes significantly to the recurring revenue streams of life sciences leaders. As noted in Thermo Fisher Scientific's 'Fourth Quarter and Full Year 2024 Financial Results' released in January 2025, the company reported full-year revenue of $42.88 billion, reflecting the industry's sustained reliance on its innovative bioscience capabilities and laboratory products to advance complex biological research.
Key Players Profiled in the Cell Culture Vessels Market
- Thermo Fisher Scientific Inc.
- Merck KGaA
- STEMCELL Technologies Canada Inc.
- Greiner Bio-One International GmbH
- Corning Incorporated
- Wilson Wolf Manufacturing, LLC
- Danaher Corporation
- WR International, LLC.
- Sartorius AG
- Cell Culture Company, LLC
Report Scope
In this report, the Global Cell Culture Vessels Market has been segmented into the following categories:Cell Culture Vessels Market, by Type:
- Reusable
- Single use
Cell Culture Vessels Market, by Product:
- Bags
- Flasks
- Plates
- Bottles
- Others
Cell Culture Vessels Market, by End Use:
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- CMOs & CROs
Cell Culture Vessels Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cell Culture Vessels Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Cell Culture Vessels market report include:- Thermo Fisher Scientific Inc.
- Merck KGaA
- STEMCELL Technologies Canada Inc.
- Greiner Bio-One International GmbH
- Corning Incorporated
- Wilson Wolf Manufacturing, LLC
- Danaher Corporation
- WR International, LLC.
- Sartorius AG
- Cell Culture Company, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
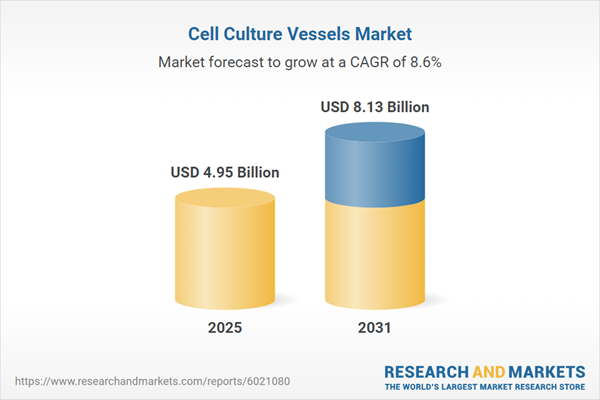
| Estimated Market Value ( USD | $ 4.95 Billion |
| Forecasted Market Value ( USD | $ 8.13 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |