Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite this growth, the market faces significant hurdles due to the high costs of advanced immunotherapeutic regimens, which restrict patient access in developing nations with limited healthcare budgets. Strict reimbursement policies and the substantial financial strain on healthcare systems frequently hinder the widespread uptake of these modern therapies, subsequently stalling revenue progress in critical emerging markets. As a result, although clinical demand for effective treatments remains strong, economic obstacles continue to impede the full commercial realization of these therapeutic innovations.
Market Drivers
The introduction and uptake of advanced immunotherapies and targeted agents are fundamentally transforming the treatment framework for small cell lung cancer. Regulatory bodies are increasingly authorizing innovative therapies that focus on specific biological pathways, providing renewed options for patients who have progressed past standard chemotherapy. This evolution is highlighted by the emergence of bispecific T-cell engagers targeting Delta-like ligand 3, representing a major shift from traditional care models. For instance, Amgen reported in a May 2024 press release that the FDA granted accelerated approval to IMDELLTRA (tarlatamab-dlle) following clinical trials that showed a median response duration of 9.7 months in patients with extensive-stage disease, a milestone that validates new mechanisms of action and spurs further clinical adoption.In parallel with these regulatory achievements, the market is propelled by significant corporate investments and strategic acquisitions designed to broaden oncology portfolios. Pharmaceutical companies are actively engaging in mergers to secure promising investigational drugs, particularly those utilizing immune engagement and precise tumor targeting.
As noted in a January 2024 press release, Merck agreed to acquire Harpoon Therapeutics for a total equity value of approximately $680 million to incorporate novel T-cell engagers into their development pipeline. These strategic financial actions ensure a steady flow of capital into research and development, speeding up the journey from clinical trials to the marketplace. Moreover, the American Cancer Society noted in 2024 that small cell lung cancer comprises about 10% to 15% of all lung cancer cases, emphasizing the urgent need for sustained therapeutic innovation.
Market Challenges
The steep prices attached to novel immunotherapeutic regimens serve as a major constraint on the Global Small Cell Lung Cancer Therapeutics Market. These high costs establish formidable economic hurdles that obstruct widespread adoption, especially in areas with restricted healthcare funding. Facing budgetary limitations, national health systems and payers often enforce rigorous reimbursement protocols that either delay or refuse access to costly treatments. Consequently, medical providers are frequently compelled to utilize older, generic chemotherapy alternatives instead of advanced agents, which directly limits potential revenue opportunities for pharmaceutical developers in price-sensitive regions.The consequences of this financial toxicity are highlighted by recent industry data regarding patient affordability. According to the American Cancer Society’s 2024 report, approximately 58 percent of cancer patients experienced financial difficulties related to treatment costs and insurance deductibles. This economic pressure restricts the number of patients able to afford high-value therapies, thereby significantly shrinking the overall market volume. Ultimately, the gap between the clinical necessity for better treatments and their economic feasibility continues to retard the commercial growth of these therapeutics.
Market Trends
The incorporation of immunotherapy combinations as a standard of care is extending beyond extensive-stage disease to include limited-stage small cell lung cancer, creating new treatment models for earlier intervention. This trend entails administering immune checkpoint inhibitors alongside concurrent chemoradiotherapy to enhance survival rates in patients with curative potential. In June 2024, AstraZeneca announced that results from the Phase III ADRIATIC trial presented at ASCO showed that durvalumab reduced the risk of death by 27% compared to placebo in patients with limited-stage disease who had not progressed after chemoradiotherapy. This broadening of immunotherapy indications marks a pivotal market shift, establishing a new care standard for a patient group that has experienced few therapeutic breakthroughs in recent decades.Concurrently, the rise of DLL3-targeting antibody-drug conjugates is establishing a potent cytotoxic approach that functions differently from bispecific T-cell engagers. Unlike therapies that engage the immune system, these conjugates employ specific antibodies to transport chemotherapy payloads directly to tumor cells expressing Delta-like ligand 3, effectively bypassing resistance issues often seen with systemic chemotherapy. In September 2024, Daiichi Sankyo reported that an interim analysis of the Phase 2 IDeate-Lung01 trial showed ifinatamab deruxtecan achieved a confirmed objective response rate of 54.8% in patients receiving the 12 mg/kg dose. This progress highlights the increasing feasibility of antibody-drug conjugates as a precision-targeted strategy for treating pretreated extensive-stage disease.
Key Players Profiled in the Small Cell Lung Cancer Therapeutics Market
- F. Hoffmann-La Roche Ltd.
- Novartis AG
- Merck & Co., Inc.
- Sun Pharmaceutical Industries Ltd.
- Lupin Limited
- Eli Lilly and Company
- Aurobindo Pharma Limited
- Bayer AG
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
Report Scope
In this report, the Global Small Cell Lung Cancer Therapeutics Market has been segmented into the following categories:Small Cell Lung Cancer Therapeutics Market, by Therapy Type:
- Immunotherapy
- Targeted Therapy
- Chemotherapy
Small Cell Lung Cancer Therapeutics Market, by Drug Type:
- Atezolizumab
- Topotecan
- Lurbinectedin
- Durvalumab
- Methotrexate side
- Pembrolizumab
Small Cell Lung Cancer Therapeutics Market, by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Small Cell Lung Cancer Therapeutics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Small Cell Lung Cancer Therapeutics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Small Cell Lung Cancer Therapeutics market report include:- F. Hoffmann-La Roche Ltd.
- Novartis AG
- Merck & Co., Inc.
- Sun Pharmaceutical Industries Ltd.
- Lupin Limited
- Eli Lilly and Company
- Aurobindo Pharma Limited
- Bayer AG
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
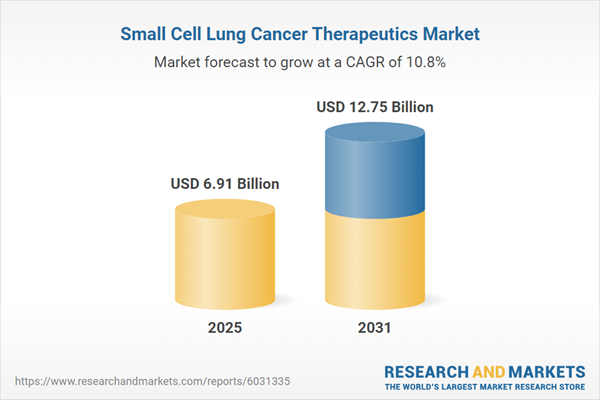
| Estimated Market Value ( USD | $ 6.91 Billion |
| Forecasted Market Value ( USD | $ 12.75 Billion |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |