Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite this positive momentum, the market encounters significant hurdles related to the high price of premium biological solutions and rigorous reimbursement protocols imposed by healthcare payers. Surgeons and hospitals often face inconsistent insurance coverage for advanced treatments like stem cell therapies and growth factors, creating financial pressure to select more affordable alternatives. Consequently, these economic constraints limit the widespread commercial adoption of newer, high-end spinal biologics, effectively slowing the penetration of premium products within the broader medical landscape.
Market Drivers
The escalating prevalence of degenerative spinal disorders among an aging global population serves as a major engine for market growth. As demographic shifts lead to a higher incidence of conditions requiring spinal fusion, there is a sustained and increasing need for effective osteoinductive agents to ensure successful bone healing in compromised patients. This trend is evidenced by robust financial performance in the sector; for instance, Orthofix Medical Inc. reported a 9% increase in net sales for their Bone Growth Therapies segment in their November 2024 earnings release. Similarly, Stryker’s October 2024 operating results showed a 10.7% rise in Orthopaedics and Spine net sales, underscoring a healthy recovery in procedural volumes that supports widespread industry demand.Concurrently, technological innovation in synthetic scaffolds is redefining clinical practices by lessening the need for autografts. Surgeons are increasingly turning to next-generation bone grafts that provide superior handling characteristics and predictable fusion results without the morbidity risks associated with traditional bone harvesting. This transition toward advanced matrices is fueling rapid adoption of products designed to replicate natural bone structure. A prime example is Kuros Biosciences, which reported in August 2024 that direct sales of its MagnetOs bone graft surged by 159% in the first half of the year, demonstrating a significant market shift toward scientifically advanced synthetic solutions that improve surgical efficiency.
Market Challenges
The sector faces substantial obstacles due to the high acquisition costs of premium biological products coupled with stringent reimbursement policies. Healthcare institutions, often operating under tight financial constraints, frequently find it difficult to justify the expenditure required for expensive bone morphogenetic proteins or cell-based matrices when more affordable alternatives, such as synthetics, are available. As a result, hospital procurement teams often limit surgeon access to these high-end items to ensure financial sustainability, which in turn restricts the revenue growth potential for advanced product categories.These financial challenges are intensified by reductions in payment rates and policy changes from major payers. According to the North American Spine Society, the Centers for Medicare & Medicaid Services enacted a nearly 3.4 percent reduction in the physician fee schedule conversion factor in 2024, creating a tougher economic landscape for complex spinal surgeries. Such cuts in reimbursement discourage the use of expensive biologics as providers attempt to lower overhead costs per procedure, ultimately dampening the adoption of premium solutions and steering the market toward commoditized, lower-margin options.
Market Trends
The market is being transformed by the emergence of peptide-enhanced osteoinductive materials, which introduce synthetic bioactive agents designed to mimic the cell-binding domains of collagen for accelerated bone repair. In contrast to traditional growth factors that may present safety concerns or high costs, these peptide-based innovations directly stimulate osteoblasts, providing a targeted approach for spinal fusion. This clinical efficacy is driving regulatory success and commercial validation; for example, Cerapedics Inc. announced in June 2025 that the pivotal study for its PearlMatrix graft showed it achieved fusion in over twice as many patients at six months compared to local autograft, highlighting the potent potential of this new biologics class.Simultaneously, the evolution of next-generation demineralized bone matrix carriers is meeting the urgent need for better graft containment and handling during complex surgeries. Manufacturers are moving away from standard putty or particulate formulations toward advanced fiber-based carriers that offer superior wicking properties and interconnected porosity, ensuring graft stability during minimally invasive procedures. This innovation is generating significant revenue growth for key players; Bioventus Inc. reported in November 2025 that its Surgical Solutions segment achieved $50.2 million in net sales, a 9.3% increase attributed largely to the strong adoption of these advanced bone graft substitutes.
Key Players Profiled in the Spine Biologics Market
- Medtronic
- Stryker
- NuVasive
- Zimmer Biomet
- SeaSpine
- DePuy Synthes
- Orthofix
- Globus Medical
- RTI Surgical
- Baxter
Report Scope
In this report, the Global Spine Biologics Market has been segmented into the following categories:Spine Biologics Market, by Product:
- Spinal Allografts
- Bone Graft Substitutes
- Cell-based Matrix
Spine Biologics Market, by Surgery:
- Anterior Cervical Discectomy and Fusion (ACDF)
- Transforaminal Lumbar Interbody Fusion (TLIF)
- Posterior Lumbar Interbody Fusion (PLIF)
- Anterior Lumbar Interbody Fusion (ALIF)
- Lateral Lumbar Interbody Fusion (LLIF)
Spine Biologics Market, by End User:
- Hospitals
- Outpatient Facilities
Spine Biologics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Spine Biologics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Spine Biologic market report include:- Medtronic
- Stryker
- NuVasive
- Zimmer Biomet
- SeaSpine
- DePuy Synthes
- Orthofix
- Globus Medical
- RTI Surgical
- Baxter
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
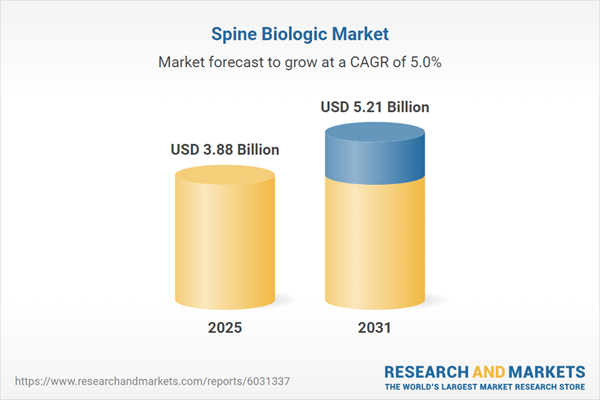
| Estimated Market Value ( USD | $ 3.88 Billion |
| Forecasted Market Value ( USD | $ 5.21 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |